Long-term functional outcome in adult prison inmates with ADHD receiving OROS-methylphenidate
- PMID: 22526730
- PMCID: PMC3491195
- DOI: 10.1007/s00406-012-0317-8
Long-term functional outcome in adult prison inmates with ADHD receiving OROS-methylphenidate
Abstract
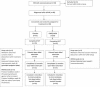
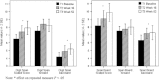
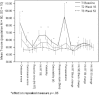
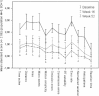
In a recent randomized, double-blind, placebo-controlled trial, we established a robust efficacy (Cohen's d = 2.17) of osmotic release oral system-methylphenidate (OROS-methylphenidate) delivered 72 mg daily for 5 weeks versus placebo on attention deficit hyperactivity disorder (ADHD) symptoms, global severity and global functioning in 30 adult male prison inmates with ADHD and coexisting disorders. Outcomes continued to improve during the subsequent 47-week open-label extension with OROS-methylphenidate delivered at a flexible daily dosage of up to 1.3 mg/kg body weight. In the present study, we evaluated long-term effectiveness and maintenance of improvement over the cumulated 52-week trial on cognition, motor activity, institutional behaviour and quality of life. Post hoc, we explored the associations between investigators' and self-ratings of ADHD symptoms and between ratings of symptoms and functioning, respectively. Outcomes, calculated by repeated measures ANOVA, improved from baseline until week 16, with maintenance or further improvement until week 52. Both verbal and visuospatial working memory, and abstract verbal reasoning improved significantly over time, as well as several cognition-related measures and motor activity. No substance abuse was detected and a majority of participants took part in psychosocial treatment programmes. The quality of life domains of Learning, and Goals and values improved over time; the latter domain was at open-label endpoint significantly related to improvements in attention. Investigators' and self-ratings of ADHD symptoms, as well as global symptom severity related most significantly to global functioning at week 52. Finally, investigators' and self-ratings of ADHD symptoms associated significantly at baseline with increasing convergence over time.
Figures
References
-
- Kooij SJJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, Edvinsson D, Fayyad J, Foeken K, Fitzgerald M, Gaillac V, Ginsberg Y, Henry C, Krause J, Lensing MB, Manor I, Niederhofer H, Nunes-Filipe C, Ohlmeier MD, Oswald P, Pallanti S, Pehlivanidis A, Ramos-Quiroga JA, Rastam M, Ryffel-Rawak D, Stes S, Asherson P. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. 2010;10:67. doi: 10.1186/1471-244X-10-67. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

