C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats
- PMID: 22526887
- PMCID: PMC3448155
- DOI: 10.1113/jphysiol.2012.232157
C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats
Abstract
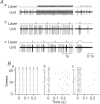
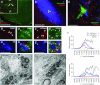
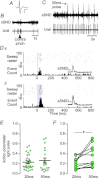
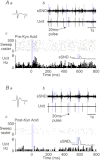
C1 neurons activate sympathetic tone and stimulate the hypothalamic–pituitary–adrenal axis in circumstances such as pain, hypoxia or hypotension. They also innervate pontine noradrenergic cell groups, including the locus coeruleus (LC) and A5. Activation of C1 neurons reportedly inhibits LC neurons; however, because these neurons are glutamatergic and have excitatory effects elsewhere, we re-examined the effect of C1 activation on pontine noradrenergic neurons (LC and A5) using a more selective method. Using a lentivirus that expresses channelrhodopsin2 (ChR2) under the control of the artificial promoter PRSx8, we restricted ChR2 expression to C1 neurons (67%), retrotrapezoid nucleus neurons (20%) and cholinergic neurons (13%). The LC contained ChR2-positive terminals that formed asymmetric synapses and were immunoreactive for vesicular glutamate transporter type 2. Low-frequency photostimulation of ChR2-expressing neurons activated LC (38 of 65; 58%) and A5 neurons (11 of 16; 69%) and sympathetic nerve discharge. Locus coeruleus and A5 inhibition was not seen unless preceded by excitation. Locus coeruleus activation was eliminated by intracerebroventricular kynurenic acid. Stimulation of ChR2-expressing neurons at 20 Hz produced modest increases in LC and A5 neuronal discharge. In additional rats, the retrotrapezoid nucleus region was destroyed with substance P–saporin prior to lentivirus injection into the rostral ventrolateral medulla, increasing the proportion of C1 ChR2-expressing neurons (83%). Photostimulation in these rats activated the same proportion of LC and A5 neurons as in control rats but produced no effect on sympathetic nerve discharge owing to the destruction of bulbospinal C1 neurons. In conclusion, low-frequency stimulation of C1 neurons activates pontine noradrenergic neurons and sympathetic nerve discharge, possibly via the release of glutamate from monosynaptic C1 inputs.
Figures




References
-
- Andrade R, Aghajanian GK. Single cell activity in the noradrenergic A-5 region: responses to drugs and peripheral manipulations of blood pressure. Brain Res. 1982;242:125–135. - PubMed
-
- Astier B, Vanbockstaele EJ, Aston-Jones G, Pieribone VA. Anatomical evidence for multiple pathways leading from the rostral ventrolateral medulla (nucleus paragigantocellularis) to the locus-coeruleus in rat. Neurosci Lett. 1990;118:141–146. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

