A new taxonomy for stakeholder engagement in patient-centered outcomes research
- PMID: 22528615
- PMCID: PMC3403141
- DOI: 10.1007/s11606-012-2037-1
A new taxonomy for stakeholder engagement in patient-centered outcomes research
Abstract
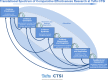
Despite widespread agreement that stakeholder engagement is needed in patient-centered outcomes research (PCOR), no taxonomy exists to guide researchers and policy makers on how to address this need. We followed an iterative process, including several stages of stakeholder review, to address three questions: (1) Who are the stakeholders in PCOR? (2) What roles and responsibilities can stakeholders have in PCOR? (3) How can researchers start engaging stakeholders? We introduce a flexible taxonomy called the 7Ps of Stakeholder Engagement and Six Stages of Research for identifying stakeholders and developing engagement strategies across the full spectrum of research activities. The path toward engagement will not be uniform across every research program, but this taxonomy offers a common starting point and a flexible approach.
Figures
References
-
- Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: from subgroups to individuals: General principles and the example of carotid endarterectomy. Lancet. 2005;365:256–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

