Variation in use of dual-chamber implantable cardioverter-defibrillators: results from the national cardiovascular data registry
- PMID: 22529229
- PMCID: PMC8317619
- DOI: 10.1001/archinternmed.2012.394
Variation in use of dual-chamber implantable cardioverter-defibrillators: results from the national cardiovascular data registry
Abstract
Background: Among patients without an indication for a pacemaker, current evidence is inconclusive whether a dual-chamber implantable cardioverter-defibrillator (ICD) is superior to a single-chamber ICD. The current use of dual-chamber ICDs is not well characterized.
Methods: We conducted a cross-sectional study exploring hospital-level variation in the use of dual-chamber ICDs across the United States. Patients receiving a primary prevention ICD from 2006 through 2009 without a documented indication for a pacemaker were included. Multivariate hierarchical logistic regression was used to explore patient, health care provider, and physician factors related to the use of a dual-chamber device.
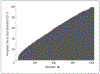
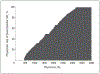
Results: Dual-chamber devices were implanted in 58% of the 87,115 patients without a pacing indication among 1293 hospitals, with hospital rates ranging from 0% in 33 centers to 100% in 109 centers. In multivariate analysis, geographic region was a strong independent predictor of dual-chamber device use, ranging from 36.4% in New England (reference region) to 66.4% in the Pacific region (odds ratio [OR], 5.25; 95% CI, 3.35-8.21). Hospital clustering was assessed using a median OR which was 3.96, meaning that 2 identical patients at different hospitals would have nearly a 4-fold difference in their chance of receiving a dual-chamber ICD.
Conclusions: Use of dual-chamber ICDs for the primary prevention of sudden cardiac death among patients without an indication for permanent pacing varies markedly at the hospital level in the United States. This is a clear example of how practice can vary independent of patient factors.
Figures


References
-
- Bardy GH, Lee KL, Mark DB, et al.; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. - PubMed
-
- Kadish A, Dyer A, Daubert JP, et al.; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, et al.; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. - PubMed
-
- McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352(3):222–224. - PubMed
-
- Centers for Medicare and Medicaid Services. National coverage decision for implantable automatic defibrillators. http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?N.... Accessed January 9, 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

