OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer
- PMID: 22529265
- PMCID: PMC3646321
- DOI: 10.1200/JCO.2012.42.0505
OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer
Abstract
Purpose: This randomized, multicenter, blinded, placebo-controlled phase III trial tested the efficacy and safety of bevacizumab (BV) with gemcitabine and carboplatin (GC) compared with GC in platinum-sensitive recurrent ovarian, primary peritoneal, or fallopian tube cancer (ROC).
Patients and methods: Patients with platinum-sensitive ROC (recurrence ≥ 6 months after front-line platinum-based therapy) and measurable disease were randomly assigned to GC plus either BV or placebo (PL) for six to 10 cycles. BV or PL, respectively, was then continued until disease progression. The primary end point was progression-free survival (PFS) by RECIST; secondary end points were objective response rate, duration of response (DOR), overall survival, and safety.
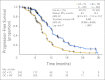
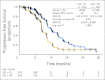
Results: Overall, 484 patients were randomly assigned. PFS for the BV arm was superior to that for the PL arm (hazard ratio [HR], 0.484; 95% CI, 0.388 to 0.605; log-rank P < .0001); median PFS was 12.4 v 8.4 months, respectively. The objective response rate (78.5% v 57.4%; P < .0001) and DOR (10.4 v 7.4 months; HR, 0.534; 95% CI, 0.408 to 0.698) were significantly improved with the addition of BV. No new safety concerns were noted. Grade 3 or higher hypertension (17.4% v < 1%) and proteinuria (8.5% v < 1%) occurred more frequently in the BV arm. The rates of neutropenia and febrile neutropenia were similar in both arms. Two patients in the BV arm experienced GI perforation after study treatment discontinuation.
Conclusion: GC plus BV followed by BV until progression resulted in a statistically significant improvement in PFS compared with GC plus PL in platinum-sensitive ROC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Gynaecological cancer: OCEANS' three: the ovarian job.Nat Rev Clin Oncol. 2012 May 15;9(6):305. doi: 10.1038/nrclinonc.2012.84. Nat Rev Clin Oncol. 2012. PMID: 22585001 No abstract available.
-
Improvement in progression-free survival in OCEANS bevacizumab arm: a critical point of view.J Clin Oncol. 2013 Jan 1;31(1):166-7. doi: 10.1200/JCO.2012.44.6237. Epub 2012 Nov 19. J Clin Oncol. 2013. PMID: 23169499 No abstract available.
References
-
- Siegel R, Naishadham MA, Jemal A. Cancer statistics, 2012. CA Can J Clin. 2012;62:10–29. - PubMed
-
- World Health Organization. International Agency for Research on Cancer: GLOBOCAN 2008 database—Summary table by cancer. http://globocan.iarc.fr/
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. - PubMed
-
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. - PubMed
-
- Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

