Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors
- PMID: 22529266
- PMCID: PMC5950496
- DOI: 10.1200/JCO.2011.36.8282
Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors
Abstract
Purpose: To determine the maximum-tolerated dose (MTD) and assess safety, pharmacokinetics, pharmacodynamics, and evidence of antitumor activity of RO4929097, a gamma secretase inhibitor of Notch signaling in patients with advanced solid malignancies.
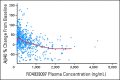
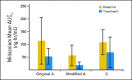
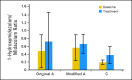
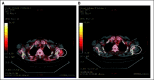
Patients and methods: Patients received escalating doses of RO4929097 orally on two schedules: (A) 3 consecutive days per week for 2 weeks every 3 weeks; (B) 7 consecutive days every 3 weeks. To assess reversible CYP3A4 autoinduction, the expanded part of the study tested three dosing schedules: (B) as above; modified A, 3 consecutive d/wk for 3 weeks; and (C) continuous daily dosing. Positron emission tomography scans with [(18)F]fluorodeoxyglucose (FDG-PET) were used to assess tumor metabolic effects.
Results: Patients on schedule A (n = 58), B (n = 47), and C (n = 5; expanded cohort) received 302 cycles of RO4929097. Common grade 1 to 2 toxicities were fatigue, thrombocytopenia, fever, rash, chills, and anorexia. Transient grade 3 hypophosphatemia (dose-limiting toxicity, one patient) and grade 3 pruritus (two patients) were observed at 27 mg and 60 mg, respectively; transient grade 3 asthenia was observed on schedule A at 80 mg (one patient). Tumor responses included one partial response in a patient with colorectal adenocarcinoma with neuroendocrine features, one mixed response (stable disease) in a patient with sarcoma, and one nearly complete FDG-PET response in a patient with melanoma. Effect on CYP3A4 induction was observed.
Conclusion: RO4929097 was well tolerated at 270 mg on schedule A and at 135 mg on schedule B; the safety of schedule C has not been fully evaluated. Further studies are warranted on the basis of a favorable safety profile and preliminary evidence of clinical antitumor activity.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures






Comment in
-
Moving forward one Notch at a time.J Clin Oncol. 2012 Jul 1;30(19):2291-3. doi: 10.1200/JCO.2012.42.3277. Epub 2012 May 7. J Clin Oncol. 2012. PMID: 22564999 No abstract available.
References
-
- Miele L, Miao H, Nickoloff BJ: NOTCH signaling as a novel cancer therapeutic target Curr Cancer Drug Targets 6:313–323,2006 - PubMed
-
- Bolós V, Grego-Bessa J, de la Pompa JL: Notch signaling in development and cancer Endocr Rev 28:339–363,2007 - PubMed
-
- Bray SJ: Notch signalling: A simple pathway becomes complex Nat Rev Mol Cell Biol 7:678–689,2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

