Dependence of Wilms tumor cells on signaling through insulin-like growth factor 1 in an orthotopic xenograft model targetable by specific receptor inhibition
- PMID: 22529373
- PMCID: PMC3356645
- DOI: 10.1073/pnas.1105034109
Dependence of Wilms tumor cells on signaling through insulin-like growth factor 1 in an orthotopic xenograft model targetable by specific receptor inhibition
Abstract
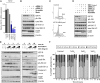
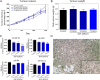
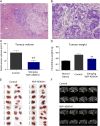
We have previously demonstrated an increased DNA copy number and expression of IGF1R to be associated with poor outcome in Wilms tumors. We have now tested whether inhibiting this receptor may be a useful therapeutic strategy by using a panel of Wilms tumor cell lines. Both genetic and pharmacological targeting resulted in inhibition of downstream signaling through PI3 and MAP kinases, G(1) cell cycle arrest, and cell death, with drug efficacy dependent on the levels of phosphorylated IGF1R. These effects were further associated with specific gene expression signatures reflecting pathway inhibition, and conferred synergistic chemosensitisation to doxorubicin and topotecan. In the in vivo setting, s.c. xenografts of WiT49 cells resembled malignant rhabdoid tumors rather than Wilms tumors. Treatment with an IGF1R inhibitor (NVP-AEW541) showed no discernable antitumor activity and no downstream pathway inactivation. By contrast, Wilms tumor cells established orthotopically within the kidney were histologically accurate and exhibited significantly elevated insulin-like growth factor-mediated signaling, and growth was significantly reduced on treatment with NVP-AEW541 in parallel with signaling pathway ablation. As a result of the paracrine effects of enhanced IGF2 expression in Wilms tumor, this disease may be acutely dependent on signaling through the IGF1 receptor, and thus treatment strategies aimed at its inhibition may be useful in the clinic. Such efficacy may be missed if only standard ectopic models are considered as a result of an imperfect recapitulation of the specific tumor microenvironment.
Conflict of interest statement
Conflict of interest statement: V.R., S.J., and F.H. are employees of Novartis Pharma.
Figures




References
-
- Pastore G, et al. Malignant renal tumours incidence and survival in European children (1978-1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2103–2114. - PubMed
-
- Kalapurakal JA, et al. Management of Wilms’ tumour: Current practice and future goals. Lancet Oncol. 2004;5:37–46. - PubMed
-
- Natrajan R, et al. Blastemal expression of type I insulin-like growth factor receptor in Wilms’ tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006;66:11148–11155. - PubMed
-
- Rainier S, et al. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

