Influenza A virus entry into cells lacking sialylated N-glycans
- PMID: 22529385
- PMCID: PMC3358892
- DOI: 10.1073/pnas.1200987109
Influenza A virus entry into cells lacking sialylated N-glycans
Abstract
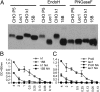
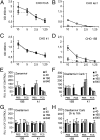
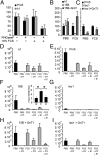
Influenza A virus (IAV) enters host cells after attachment of its hemagglutinin (HA) to surface-exposed sialic acid. Sialylated N-linked glycans have been reported to be essential for IAV entry [Chu VC, Whittaker GR (2004) Proc Natl Acad Sci USA 102:18153-18158], thereby implicating the requirement for proteinaceous receptors in IAV entry. Here we show, using different N-acetylglucosaminyl transferase 1 (GnT1)-deficient cells, that N-linked sialosides can mediate, but are not required for, entry of IAV. Entry into GnT1-deficient cells was fully dependent on sialic acid. Although macropinocytic entry appeared to be affected by the absence of sialylated N-glycans, dynamin-dependent entry was not affected at all. However, binding of HA to GnT1-deficient cells and subsequent entry of IAV were reduced by the presence of serum, which could be reversed by back-transfection of a GnT1-encoding plasmid. The inhibitory effect of serum was significantly increased by inhibition of the viral receptor-destroying enzyme neuraminidase (NA). Our results indicate that decoy receptors on soluble serum factors compete with cell surface receptors for binding to HA in the absence of sialylated N-glycans at the cell surface. This competition is particularly disturbed by the additional presence of NA inhibitors, resulting in strongly reduced IAV entry. Our results indicate that the balance between HA and NA is important not only for virion release, but also for entry into cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

