HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells
- PMID: 22529395
- PMCID: PMC3358844
- DOI: 10.1073/pnas.1201104109
HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells
Abstract
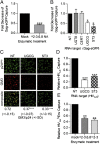
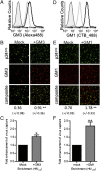
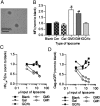
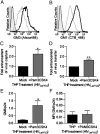
The interaction between HIV and dendritic cells (DCs) is an important early event in HIV-1 pathogenesis that leads to efficient viral dissemination. Here we demonstrate a HIV gp120-independent DC capture mechanism that uses virion-incorporated host-derived gangliosides with terminal α2-3-linked sialic acid linkages. Using exogenously enriched virus and artificial liposome particles, we demonstrate that both α2-3 gangliosides GM1 and GM3 are capable of mediating this interaction when present in the particle at high levels. In the absence of overexpression, GM3 is the primary ligand responsible for this capture mechanism, because siRNA depletion of GM3 but not GM1 from the producer cell and hence virions, resulted in a dramatic decrease in DC capture. Furthermore, HIV-1 capture by DCs was competitively inhibited by targeting virion-associated GM3, but was unchanged by targeting GM1. Finally, virions were derived from monocytoid THP-1 cells that constitutively display low levels of GM1 and GM3, or from THP-1 cells induced to express high surface levels of GM1 and GM3 upon stimulation with the TLR2/1 ligand Pam3CSK4. Compared with untreated THP-1 cells, virus produced from Pam3CSK4-stimulated THP-1 cells incorporated higher levels of GM3, but not GM1, and showed enhanced DC capture and trans-infection. Our results identify a unique HIV-1 DC attachment mechanism that is dependent on a host-cell-derived ligand, GM3, and is a unique example of pathogen mimicry of host-cell recognition pathways that drive virus capture and dissemination in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- UNAIDS . Geneva: UNAIDS; 2008. 2008 Report on the global AIDS epidemic.
-
- Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. - PubMed
-
- Cameron PU, et al. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. - PubMed
-
- McDonald D, et al. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

