A meta-analysis of probiotic efficacy for gastrointestinal diseases
- PMID: 22529959
- PMCID: PMC3329544
- DOI: 10.1371/journal.pone.0034938
A meta-analysis of probiotic efficacy for gastrointestinal diseases
Abstract
Background: Meta-analyses on the effects of probiotics on specific gastrointestinal diseases have generally shown positive effects on disease prevention and treatment; however, the relative efficacy of probiotic use for treatment and prevention across different gastrointestinal diseases, with differing etiology and mechanisms of action, has not been addressed.
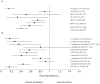
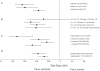
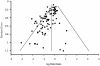
Methods/principal findings: We included randomized controlled trials in humans that used a specified probiotic in the treatment or prevention of Pouchitis, Infectious diarrhea, Irritable Bowel Syndrome, Helicobacter pylori, Clostridium difficile Disease, Antibiotic Associated Diarrhea, Traveler's Diarrhea, or Necrotizing Enterocolitis. Random effects models were used to evaluate efficacy as pooled relative risks across the eight diseases as well as across probiotic species, single vs. multiple species, patient ages, dosages, and length of treatment. Probiotics had a positive significant effect across all eight gastrointestinal diseases with a relative risk of 0.58 (95% (CI) 0.51-0.65). Six of the eight diseases: Pouchitis, Infectious diarrhea, Irritable Bowel Syndrome, Helicobacter pylori, Clostridium difficile Disease, and Antibiotic Associated Diarrhea, showed positive significant effects. Traveler's Diarrhea and Necrotizing Enterocolitis did not show significant effects of probiotcs. Of the 11 species and species mixtures, all showed positive significant effects except for Lactobacillus acidophilus, Lactobacillus plantarum, and Bifidobacterium infantis. Across all diseases and probiotic species, positive significant effects of probiotics were observed for all age groups, single vs. multiple species, and treatment lengths.
Conclusions/significance: Probiotics are generally beneficial in treatment and prevention of gastrointestinal diseases. Efficacy was not observed for Traveler's Diarrhea or Necrotizing Enterocolitis or for the probiotic species L. acidophilus, L. plantarum, and B. infantis. When choosing to use probiotics in the treatment or prevention of gastrointestinal disease, the type of disease and probiotic species (strain) are the most important factors to take into consideration.
Conflict of interest statement
Figures


References
-
- McFarland LV. Meta-Analysis of Probiotics for the prevention of Antibiotic Associated Diarrhea and the Treatment of Clostridium difficile Disease. Am J Gastroenterol. 2006;101:812–822. - PubMed
-
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–82. - PubMed
-
- Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25(2):155–68. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

