Mycobacterium tuberculosis Rv3586 (DacA) is a diadenylate cyclase that converts ATP or ADP into c-di-AMP
- PMID: 22529992
- PMCID: PMC3328451
- DOI: 10.1371/journal.pone.0035206
Mycobacterium tuberculosis Rv3586 (DacA) is a diadenylate cyclase that converts ATP or ADP into c-di-AMP
Abstract
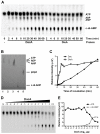
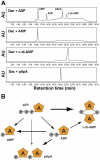
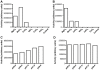
Cyclic diguanosine monophosphate (c-di-GMP) and cyclic diadenosine monophosphate (c-di-AMP) are recently identified signaling molecules. c-di-GMP has been shown to play important roles in bacterial pathogenesis, whereas information about c-di-AMP remains very limited. Mycobacterium tuberculosis Rv3586 (DacA), which is an ortholog of Bacillus subtilis DisA, is a putative diadenylate cyclase. In this study, we determined the enzymatic activity of DacA in vitro using high-performance liquid chromatography (HPLC), mass spectrometry (MS) and thin layer chromatography (TLC). Our results showed that DacA was mainly a diadenylate cyclase, which resembles DisA. In addition, DacA also exhibited residual ATPase and ADPase in vitro. Among the potential substrates tested, DacA was able to utilize both ATP and ADP, but not AMP, pApA, c-di-AMP or GTP. By using gel filtration and analytical ultracentrifugation, we further demonstrated that DacA existed as an octamer, with the N-terminal domain contributing to tetramerization and the C-terminal domain providing additional dimerization. Both the N-terminal and the C-terminal domains were essential for the DacA's enzymatically active conformation. The diadenylate cyclase activity of DacA was dependent on divalent metal ions such as Mg(2+), Mn(2+) or Co(2+). DacA was more active at a basic pH rather than at an acidic pH. The conserved RHR motif in DacA was essential for interacting with ATP, and mutation of this motif to AAA completely abolished DacA's diadenylate cyclase activity. These results provide the molecular basis for designating DacA as a diadenylate cyclase. Our future studies will explore the biological function of this enzyme in M. tuberculosis.
Conflict of interest statement
Figures
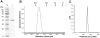



References
-
- Aziz MA, Wright A. The World Health Organization/International Union Against Tuberculosis and Lung Disease Global Project on Surveillance for Anti-Tuberculosis Drug Resistance: a model for other infectious diseases. Clin Infect Dis. 2005;41(Suppl 4):S258–262. - PubMed
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
-
- Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83:44–51. - PubMed
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

