Propylthiouracil is teratogenic in murine embryos
- PMID: 22529993
- PMCID: PMC3329451
- DOI: 10.1371/journal.pone.0035213
Propylthiouracil is teratogenic in murine embryos
Abstract
Background: Hyperthyroidism during pregnancy is treated with the antithyroid drugs (ATD) propylthiouracil (PTU) and methimazole (MMI). PTU currently is recommended as the drug of choice during early pregnancy. Yet, despite widespread ATD use in pregnancy, formal studies of ATD teratogenic effects have not been performed.
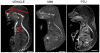
Methods: We examined the teratogenic effects of PTU and MMI during embryogenesis in mice. To span different periods of embryogenesis, dams were treated with compounds or vehicle daily from embryonic day (E) 7.5 to 9.5 or from E3.5 to E7.5. Embryos were examined for gross malformations at E10.5 or E18.5 followed by histological and micro-CT analysis. Influences of PTU on gene expression levels were examined by RNA microarray analysis.
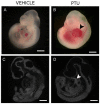
Results: When dams were treated from E7.5 to E9.5 with PTU, neural tube and cardiac abnormalities were observed at E10.5. Cranial neural tube defects were significantly more common among the PTU-exposed embryos than those exposed to MMI or vehicle. Blood in the pericardial sac, which is a feature indicative of abnormal cardiac function and/or abnormal vasculature, was observed more frequently in PTU-treated than MMI-treated or vehicle-treated embryos. Following PTU treatment, a total of 134 differentially expressed genes were identified. Disrupted genetic pathways were those associated with cytoskeleton remodeling and keratin filaments. At E 18.5, no gross malformations were evident in either ATD group, but the number of viable PTU embryos per dam at E18.5 was significantly lower from those at E10.5, indicating loss of malformed embryos. These data show that PTU exposure during embryogenesis is associated with delayed neural tube closure and cardiac abnormalities. In contrast, we did not observe structural or cardiac defects associated with MMI exposure except at the higher dose. We find that PTU exposure during embryogenesis is associated with fetal loss. These observations suggest that PTU has teratogenic potential.
Conflict of interest statement
Figures




References
-
- Weetman A. Grave's disease 1835–2002. Horm Res. 2003;59(Suppl 1):114–118. - PubMed
-
- Cooper D. Antithyroid drugs. N Engl J Med. 2005;352:905–917. - PubMed
-
- Rivkees SA. Pediatric Graves' Disease: Controversies in Management. Horm Res Paediatr. 2010;74(5):305–311. - PubMed
-
- Patil-Sisodia K, Mestman J. Graves hyperthyroidism and pregnancy: a clinical update. Endocr Pract. 2010;16:118–129. - PubMed
-
- Martin J, Hamilton B, Sutton P, Ventura S, Menacker F, et al. National Vital Statistics Reports. 7. Vol. 57. Hyattsville, MD: Centers for Disease Control and Prevention; 2009. Births: Final Data for 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

