Inhibition of diacylglycerol-sensitive TRPC channels by synthetic and natural steroids
- PMID: 22530015
- PMCID: PMC3328449
- DOI: 10.1371/journal.pone.0035393
Inhibition of diacylglycerol-sensitive TRPC channels by synthetic and natural steroids
Abstract
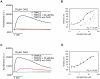
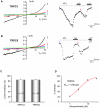
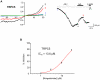
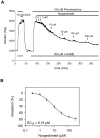
TRPC channels are a family of nonselective cation channels that regulate ion homeostasis and intracellular Ca(2+) signaling in numerous cell types. Important physiological functions such as vasoregulation, neuronal growth, and pheromone recognition have been assigned to this class of ion channels. Despite their physiological relevance, few selective pharmacological tools are available to study TRPC channel function. We, therefore, screened a selection of pharmacologically active compounds for TRPC modulating activity. We found that the synthetic gestagen norgestimate inhibited diacylglycerol-sensitive TRPC3 and TRPC6 with IC(50)s of 3-5 µM, while half-maximal inhibition of TRPC5 required significantly higher compound concentrations (>10 µM). Norgestimate blocked TRPC-mediated vasopressin-induced cation currents in A7r5 smooth muscle cells and caused vasorelaxation of isolated rat aorta, indicating that norgestimate could be an interesting tool for the investigation of TRP channel function in native cells and tissues. The steroid hormone progesterone, which is structurally related to norgestimate, also inhibited TRPC channel activity with IC(50)s ranging from 6 to 18 µM but showed little subtype selectivity. Thus, TRPC channel inhibition by high gestational levels of progesterone may contribute to the physiological decrease of uterine contractility and immunosuppression during pregnancy.
Conflict of interest statement
Figures

References
-
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. - PubMed
-
- Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney JW., Jr Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol. 2007:593–614. - PubMed
-
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

