Hypoxia modulates the expression of leucine zipper-positive MYPT1 and its interaction with protein kinase G and Rho kinases in pulmonary arterial smooth muscle cells
- PMID: 22530104
- PMCID: PMC3329079
- DOI: 10.4103/2045-8932.93548
Hypoxia modulates the expression of leucine zipper-positive MYPT1 and its interaction with protein kinase G and Rho kinases in pulmonary arterial smooth muscle cells
Abstract
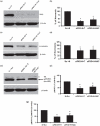
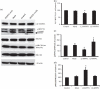
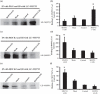
We have shown previously that acute hypoxia downregulates protein kinase G (PKG) expression and activity in ovine fetal pulmonary vessels and pulmonary arterial smooth muscle cells (SMC). Here, we report that acute hypoxia also reduces the expression of leucinezipper-positive MYPT1 (LZ(+)MYPT1), a subunit of myosin light chain (MLC) phosphatase, in ovine fetal pulmonary arterial SMC. We found that in hypoxia, there is greater interaction between LZ(+) MYPT1 and RhoA and Rho kinase 1 (ROCK1)/Rho kinase 2 (ROCK2) and decreased interaction between LZ(+) MYPT1 and PKG, resulting in increased MLC(20) phosphorylation, a higher pMLC(20)/MLC(20) ratio and SMC contraction. In normoxic SMC PKG overexpression, LZ(+) MYPT1 expression is upregulated while PKG knockdown had an opposite effect. LZ(+) MYPT1 overexpression enhanced the interaction between PKG and LZ(+) MYPT1. Overexpression of a mutant LZ(-) MYPT1 isoform in SMC mimicked the effects of acute hypoxia and decreased pMLC(20)/MLC(20) ratio. Collectively, our data suggest that hypoxia downregulates LZ(+) MYPT1 expression by suppressing PKG levels, reduces the interaction of LZ(+) MYPT1 with PKG and promotes LZ(+) MYPT1 interaction with RhoA or ROCK1/ROCK2, thereby promoting pulmonary arterial SMC contraction.
Keywords: cGMP; hypoxia-induced pulmonary hypertension; pulmonary vasoconstriction; signal transduction.
Conflict of interest statement
Figures






Similar articles
-
Differential phosphorylation of LZ+/LZ- MYPT1 isoforms regulates MLC phosphatase activity.Arch Biochem Biophys. 2014 Nov 15;562:37-42. doi: 10.1016/j.abb.2014.08.011. Epub 2014 Aug 26. Arch Biochem Biophys. 2014. PMID: 25168281
-
Degradation of leucine zipper-positive isoform of MYPT1 may contribute to development of nitrate tolerance.Cardiovasc Res. 2010 Apr 1;86(1):151-9. doi: 10.1093/cvr/cvp376. Epub 2009 Nov 25. Cardiovasc Res. 2010. PMID: 19939965
-
MYPT1 isoforms expressed in HEK293T cells are differentially phosphorylated after GTPγS treatment.J Smooth Muscle Res. 2016;52(0):66-77. doi: 10.1540/jsmr.52.66. J Smooth Muscle Res. 2016. PMID: 27725371 Free PMC article.
-
The potential role of MLC phosphatase and MAPK signalling in the pathogenesis of vascular dysfunction in heart failure.J Cell Mol Med. 2008 Dec;12(6A):2158-64. doi: 10.1111/j.1582-4934.2008.00536.x. J Cell Mol Med. 2008. PMID: 19120700 Free PMC article. Review.
-
Regulation of Smooth Muscle Myosin Light Chain Phosphatase by Multisite Phosphorylation of the Myosin Targeting Subunit, MYPT1.Cardiovasc Hematol Disord Drug Targets. 2018;18(1):4-13. doi: 10.2174/1871529X18666180326120638. Cardiovasc Hematol Disord Drug Targets. 2018. PMID: 29577868 Review.
Cited by
-
Intrapulmonary arterial contraction assay reveals region-specific deregulation of vasoreactivity to lung injuries.Am J Physiol Lung Cell Mol Physiol. 2023 Aug 1;325(2):L114-L124. doi: 10.1152/ajplung.00293.2022. Epub 2023 Jun 6. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37278410 Free PMC article.
-
T18/S19 diphosphorylation of myosin regulatory light chain impairs pulmonary artery relaxation in monocrotaline-induced pulmonary hypertensive rats.Pflugers Arch. 2023 Sep;475(9):1097-1112. doi: 10.1007/s00424-023-02836-6. Epub 2023 Jul 8. Pflugers Arch. 2023. PMID: 37422604
-
Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion.EMBO J. 2014 Sep 17;33(18):2040-56. doi: 10.15252/embj.201488641. Epub 2014 Jul 28. EMBO J. 2014. PMID: 25069772 Free PMC article.
-
Recent Progress in Research on the Pathogenesis of Pulmonary Thromboembolism: An Old Story with New Perspectives.Biomed Res Int. 2017;2017:6516791. doi: 10.1155/2017/6516791. Epub 2017 Apr 6. Biomed Res Int. 2017. PMID: 28484717 Free PMC article. Review.
-
Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation.Int J Mol Sci. 2021 Aug 11;22(16):8622. doi: 10.3390/ijms22168622. Int J Mol Sci. 2021. PMID: 34445328 Free PMC article. Review.
References
-
- Mark Evans A, Ward JP. Hypoxic pulmonary vasoconstriction-invited article. AdvExp Med Biol. 2009;648:351–60. - PubMed
-
- Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, et al. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J BiolChem. 1992;267:14662–8. - PubMed
-
- Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol Cell Physiol. 2000;279:C1722–32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

