Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway
- PMID: 22530672
- PMCID: PMC6493617
- DOI: 10.1111/j.1755-5949.2012.00319.x
Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway
Abstract
Background and purpose: Resveratrol has been regarded as a promising candidate for cancer prevention and treatment. The present study was to investigate the impact of resveratrol on the antitumor effects of temozolomide (TMZ), a standard treatment regiment of glioblastoma (GBM), in vitro and in vivo.
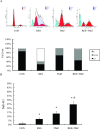
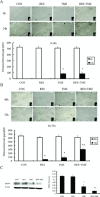
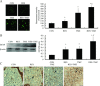
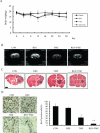
Methods and results: We found that the combination of resveratrol and TMZ significantly resulted in G(2)/M cell cycle arrest by flow cytometry, triggered a robust increase in expression of astrocyte differentiation marker glial fibrillary acid protein (GFAP), downregulated the expression of matrix metalloproteinase-9 (MMP-9) by immunohistochemistry and western blot analysis as well as inhibited cell migration by scratch wound assay. Further study revealed that TMZ in combination with resveratrol remarkably increased reactive oxygen species (ROS) production, which serves as an upstream signal for AMP-activated protein kinase (AMPK) activation. Subsequently, activated AMPK inhibited mTOR signaling and downregulated antiapoptosis protein Bcl-2, which was contributed to the additive antiproliferation effects of combination treatment. In an orthotopic xenograft model of GBM, TMZ plus resveratrol treatment significantly reduced the volume of tumor, which was confirmed by decreased expression of Ki-67, a marker of proliferation index.
Conclusions: Our findings demonstrate for the first time that resveratrol can enhance TMZ-mediated antitumor effects in GBM in vitro and in vivo, via ROS-dependent AMPK-TSC-mTOR signaling pathway.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, et al European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. - PubMed
-
- Uzzaman M, Keller G, Germano IM. Enhanced proapoptotic effects of tumor necrosis factor‐related apoptosis‐inducing ligand on temozolomide resistant glioma cells. J Neurosurg 2007;106:646–651. - PubMed
-
- Tentori L, Graziani G. Recent approaches to improve the antitumor efficacy of Temozolomide. Curr Med Chem 2009;16:245–257. - PubMed
-
- Gagliano N, Moscheni C, Torri C, et al Effect of resveratrol on matrix metalloproteinase‐2 (MMP‐2) and secreted protein acidic and rich in cysteine (SPARC) on human cultured glioblastoma cells. Biomed Pharmacother 2005;59:359–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

