Human amniotic mesenchymal stem cell-derived induced pluripotent stem cells may generate a universal source of cardiac cells
- PMID: 22530853
- PMCID: PMC3464077
- DOI: 10.1089/scd.2011.0435
Human amniotic mesenchymal stem cell-derived induced pluripotent stem cells may generate a universal source of cardiac cells
Abstract
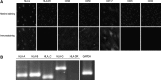
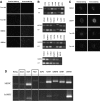
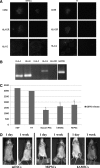
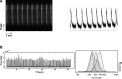
Human amniotic mesenchymal stem cells (hAMSCs) demonstrated partially pluripotent characteristics with a strong expression of Oct4 and Nanog genes and immunomodulatory properties characterized by the absence of HLA-DR and the presence of HLA-G and CD59. The hAMSCs were reprogrammed into induced pluripotent stem cells (iPSCs) that generate a promising source of universal cardiac cells. The hAMSC-derived iPSCs (MiPSCs) successfully underwent robust cardiac differentiation to generate cardiomyocytes. This study investigated 3 key properties of the hAMSCs and MiPSCs: (1) the reprogramming efficiency of the partially pluripotent hAMSCs to generate MiPSCs; (2) immunomodulatory properties of the hAMSCs and MiPSCs; and (3) the cardiac differentiation potential of the MiPSCs. The characteristic iPSC colony formation was observed within 10 days after the transduction of the hAMSCs with a single integration polycistronic vector containing 4 Yamanaka factors. Immunohistology and reverse transcription-polymerase chain reaction assays revealed that the MiPSCs expressed stem cell surface markers and pluripotency-specific genes. Furthermore, the hAMSCs and MiPSCs demonstrated immunomodulatory properties enabling successful engraftment in the SVJ mice. Finally, the cardiac differentiation of MiPSCs exhibited robust spontaneous contractility, characteristic calcium transience across the membrane, a high expression of cardiac genes and mature cardiac phenotypes, and a contractile force comparable to cardiomyocytes. Our results demonstrated that the hAMSCs are reprogrammed with a high efficiency into MiPSCs, which possess pluripotent, immunomodulatory, and precardiac properties. The MiPSC-derived cardiac cells express a c-kit cell surface marker, which may be employed to purify the cardiac cell population and enable allogeneic cardiac stem cell therapy.
Figures


References
-
- Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Shi Y. Desponts C. Do JT. Hahm HS. Scholer HR. Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

