Prodrug strategies in ocular drug delivery
- PMID: 22530907
- PMCID: PMC4407659
- DOI: 10.2174/157340612801216283
Prodrug strategies in ocular drug delivery
Abstract
Poor bioavailability of topically instilled drug is the major concern in the field of ocular drug delivery. Efflux transporters, static and dynamic ocular barriers often possess rate limiting factors for ocular drug therapy. Different formulation strategies like suspension, ointment, gels, nanoparticles, implants, dendrimers and liposomes have been employed in order to improve drug permeation and retention by evading rate limiting factors at the site of absorption. Chemical modification such as prodrug targeting various nutrient transporters (amino acids, peptide and vitamin) has evolved a great deal of interest to improve ocular drug delivery. In this review, we have discussed various prodrug strategies which have been widely applied for enhancing therapeutic efficacy of ophthalmic drugs. The purpose of this review is to provide an update on the utilization of prodrug concept in ocular drug delivery. In addition, this review will highlight ongoing academic and industrial research and development in terms of ocular prodrug design and delivery.
Figures
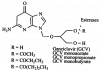
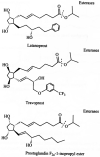





Similar articles
-
Ocular prodrugs: Attributes and challenges.Asian J Pharm Sci. 2021 Mar;16(2):175-191. doi: 10.1016/j.ajps.2020.08.002. Epub 2020 Sep 28. Asian J Pharm Sci. 2021. PMID: 33995612 Free PMC article. Review.
-
Prodrugs and nanomicelles to overcome ocular barriers for drug penetration.Expert Opin Drug Metab Toxicol. 2020 Oct;16(10):885-906. doi: 10.1080/17425255.2020.1803278. Epub 2020 Aug 23. Expert Opin Drug Metab Toxicol. 2020. PMID: 32729364 Review.
-
Ocular drug metabolism of the bioactivating antioxidant N-acetylcarnosine for vision in ophthalmic prodrug and codrug design and delivery.Drug Dev Ind Pharm. 2008 Oct;34(10):1071-89. doi: 10.1080/03639040801958413. Drug Dev Ind Pharm. 2008. PMID: 18777243
-
Advances in the use of prodrugs for drug delivery to the eye.Expert Opin Drug Deliv. 2017 Jan;14(1):49-63. doi: 10.1080/17425247.2016.1208649. Epub 2016 Jul 21. Expert Opin Drug Deliv. 2017. PMID: 27441817 Free PMC article. Review.
-
Bioactivation antioxidant and transglycating properties of N-acetylcarnosine autoinduction prodrug of a dipeptide L-carnosine in mucoadhesive drug delivery eye-drop formulation: powerful eye health application technique and therapeutic platform.Drug Test Anal. 2012 Jun;4(6):468-85. doi: 10.1002/dta.265. Epub 2011 Mar 18. Drug Test Anal. 2012. PMID: 21416634
Cited by
-
SG-SP1 Suppresses Mast Cell-Mediated Allergic Inflammation via Inhibition of FcεRI Signaling.Front Immunol. 2020 Jan 28;11:50. doi: 10.3389/fimmu.2020.00050. eCollection 2020. Front Immunol. 2020. PMID: 32063904 Free PMC article.
-
A convenient and eco-friendly cerium(III) chloride-catalysed synthesis of methoxime derivatives of aromatic aldehydes and ketones.R Soc Open Sci. 2018 May 23;5(5):180279. doi: 10.1098/rsos.180279. eCollection 2018 May. R Soc Open Sci. 2018. PMID: 29892459 Free PMC article.
-
Ocular prodrugs: Attributes and challenges.Asian J Pharm Sci. 2021 Mar;16(2):175-191. doi: 10.1016/j.ajps.2020.08.002. Epub 2020 Sep 28. Asian J Pharm Sci. 2021. PMID: 33995612 Free PMC article. Review.
-
Evaluation of Safety, Tolerability and Pharmacokinetic Characteristics of SA001 and Its Active Metabolite Rebamipide after Single and Multiple Oral Administration.Pharmaceuticals (Basel). 2023 Jan 16;16(1):132. doi: 10.3390/ph16010132. Pharmaceuticals (Basel). 2023. PMID: 36678630 Free PMC article.
-
Multivalent Carbonic Anhydrases Inhibitors.Int J Mol Sci. 2019 Oct 28;20(21):5352. doi: 10.3390/ijms20215352. Int J Mol Sci. 2019. PMID: 31661796 Free PMC article. Review.
References
-
- Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci. 2006;95(6):1177–95. - PubMed
-
- Testa B, Mayer JM. Hydrolysis in Drug and Prodrug Metabolism. Wiley-VCH Verlag GmbH; Weinheim: 2003.
-
- Kwatra D, Vaishya R, Gaudana R, Jwala J. In: Prodrugs and Targeted Delivery Towards Beller ADME Properties. Rautio Jarkko., editor. Vol. 47. Wiley-VCH; Germany: 2011. pp. 181–201.
-
- Faulkner R, Sharif NA, Orr S, Sall K, Dubiner H, Whitson JT, Moster M, Craven ER, Curtis M, Pailliotet C, Martens K, Dahlin D. Aqueous humor concentrations of bimatoprost free acid, bimatoprost and travoprost free acid in cataract surgical patients administered multiple topcal ocular doses of LUMIGAN or TRAVATAN. J. Ocul. Pharmacal. Ther. 2010;26(2):147–56. - PubMed
-
- Fukano Y, Kawazu K. Disposition and metabolism of a novel prostanoid antiglaucoma medication, tafluprost, following ocular administration to rats. Drug. Metab. Dispos. 2009;37(8):1622–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

