Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons
- PMID: 22531176
- PMCID: PMC3337979
- DOI: 10.1038/ncomms1795
Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons
Abstract
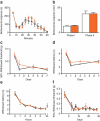
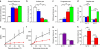
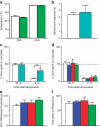
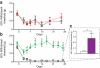
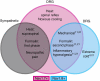
Human acute and inflammatory pain requires the expression of voltage-gated sodium channel Nav1.7 but its significance for neuropathic pain is unknown. Here we show that Nav1.7 expression in different sets of mouse sensory and sympathetic neurons underlies distinct types of pain sensation. Ablating Nav1.7 gene (SCN9A) expression in all sensory neurons using Advillin-Cre abolishes mechanical pain, inflammatory pain and reflex withdrawal responses to heat. In contrast, heat-evoked pain is retained when SCN9A is deleted only in Nav1.8-positive nociceptors. Surprisingly, responses to the hotplate test, as well as neuropathic pain, are unaffected when SCN9A is deleted in all sensory neurons. However, deleting SCN9A in both sensory and sympathetic neurons abolishes these pain sensations and recapitulates the pain-free phenotype seen in humans with SCN9A loss-of-function mutations. These observations demonstrate an important role for Nav1.7 in sympathetic neurons in neuropathic pain, and provide possible insights into the mechanisms that underlie gain-of-function Nav1.7-dependent pain conditions.
Figures
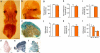
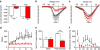
References
-
- Momin A. & Wood J. N. Sensory neuron voltage-gated sodium channels as analgesic drug targets. Curr. Opin. Neurobiol. 18, 383–388 (2008). - PubMed
-
- Dib-Hajj S. D., Yang Y. & Waxman S. G. Advances in Genetics 63, 85–110 (Elsevier, 2008). - PubMed
-
- Zimmermann K. et al.. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447, 855–858 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

