First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands
- PMID: 22531183
- PMCID: PMC3337976
- DOI: 10.1038/ncomms1802
First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands
Abstract
Bestowing chirality to metals is central in fields such as heterogeneous catalysis and modern optics. Although the bulk phase of metals is symmetric, their surfaces can become chiral through adsorption of molecules. Interestingly, even achiral molecules can lead to locally chiral, though globally racemic, surfaces. A similar situation can be obtained for metal particles or clusters. Here we report the first separation of the enantiomers of a gold cluster protected by achiral thiolates, Au(38)(SCH(2)CH(2)Ph)(24), achieved by chiral high-performance liquid chromatography. The chirality of the nanocluster arises from the chiral arrangement of the thiolates on its surface, forming 'staple motifs'. The enantiomers show mirror-image circular dichroism responses and large anisotropy factors of up to 4×10(-3). Comparison with reported circular dichroism spectra of other Au(38) clusters reveals that the influence of the ligand on the chiroptical properties is minor.
Figures
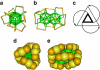

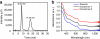

References
-
- Böhringer M., Morgenstern K., Schneider W.- D. & Berndt R. Separation of a Racemic Mixture of Two-Dimensional Molecular Clusters by Scanning Tunneling Microscopy. Angew. Chem. Int. Ed. 38, 821–823 (1999). - PubMed
-
- Chen Q., Frankel D. J. & Richardson N. V. Chemisorption induced chirality: glycine on Cu{110}. Surf. Sci. 497, 37–46 (2002).
-
- Parschau M., Romer S. & Ernst K. H. Induction of homochirality in achiral enantiomorphous monolayers. J. Am. Chem. Soc. 126, 15398–15399 (2004). - PubMed
-
- Lorenzo M. O., Baddeley C. J., Muryn C. & Raval R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 404, 376–379 (2000). - PubMed
-
- Jadzinsky P. D., Calero G., Ackerson C. J., Bushnell D. A. & Kornberg R. D. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 A resolution. Science 318, 430–433 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

