Galactosylation variations in marketed therapeutic antibodies
- PMID: 22531450
- PMCID: PMC3355481
- DOI: 10.4161/mabs.19868
Galactosylation variations in marketed therapeutic antibodies
Abstract
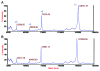
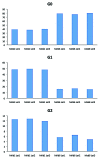
There are currently ~25 recombinant full-length IgGs (rIgGs) in the market that have been approved by regulatory agencies as biotherapeutics to treat various human diseases. Most of these are based on IgG1k framework and are either chimeric, humanized or human antibodies manufactured using either Chinese hamster ovary (CHO) cells or mouse myeloma cells as the expression system. Because CHO and mouse myeloma cells are mammalian cells, rIgGs produced in these cell lines are typically N-glycosylated at the conserved asparagine (Asn) residues in the CH2 domain of the Fc, which is also the case with serum IgGs. The Fc glycans present in these rIgGs are for the most part complex biantennary oligosaccharides with heterogeneity associated with the presence or the absence of several different terminal sugars. The major Fc glycans of rIgGs contain 0 or 1 or 2 (G0, G1 and G2, respectively) terminal galactose residues as non-reducing termini and their relative proportions may vary depending on the cell culture conditions in which they were expressed. Since glycosylation is strongly associated with antibody effector functions and terminal galactosylation may affect some of those functions, a panel of commercially available therapeutic rIgGs expressed in CHO cells and mouse myeloma cells were examined for their galactosylation patterns. The results suggest that the rIgGs expressed in CHO cells are generally less galactosylated compared to the rIgGs expressed in mouse myeloma cells. Accordingly, rIgGs produced in CHO cells tend to contain higher levels of G0 glycans compared with rIgGs produced in mouse myeloma cell lines. Despite the apparent wide variability in galactose content, adverse events or safety issues have not been associated with specific galactosylation patterns of therapeutic antibodies. Nevertheless, galactosylation may have an effect on the mechanisms of action of some therapeutic antibodies (e.g., effector pathways) and hence further studies to assess effects on product efficacy may be warranted for such antibodies. For antibodies that do not require effector functions for biological activity, however, setting a narrow specification range for galactose content may be unnecessary.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
