The zinc finger transcription factor ZKSCAN3 promotes prostate cancer cell migration
- PMID: 22531714
- PMCID: PMC3358443
- DOI: 10.1016/j.biocel.2012.04.005
The zinc finger transcription factor ZKSCAN3 promotes prostate cancer cell migration
Abstract
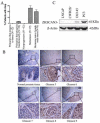
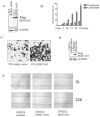
In our previous studies, ZKSCAN3 was demonstrated to be over-expressed in invasive colonic tumor cells and their liver metastases, but minimally expressed in adjacent non-transformed tissues. Further preliminary data showed that ZKSCAN3 was expressed in a majority of prostate cancer patient samples, but not in normal prostate tissues. Moreover, the ZKSCAN3 protein is highly expressed in the PC3 prostate cancer cell line, which has high metastatic potential, but little expression was observed in non-metastatic prostate cancer cell lines. Thus, we hypothesized that ZKSCAN3 could participate in tumor metastasis by regulating tumor cell migration. To test this hypothesis, ZKSCAN3 mRNA was knocked down by ZKSCAN3 specific shRNA in PC3 cells and a significant decrease in cell motility was observed. In contrast, when ZKSCAN3 cDNA was overexpressed in PC3 cells, cell detachment was observed and suspension culture induced apoptosis was greatly decreased, suggesting that ZKSCAN3 is able to enhance PC3 cell survival under anoikis stress. Additional wound healing and invasion assays showed that cell migration was enhanced by ZKSCAN3 expression. Interestingly, the ZKSCAN3 gene was amplified in 26% (5/19) of metastatic prostate cancers and 20% (1/5) of lymph node metastases, but there was no amplification found in primary prostate cancers, further supporting the role of ZKSCAN3 in tumor cell migration. In vivo studies using orthotopic tumor models indicated that overexpression of ZKSCAN3 significantly enhanced tumorigenicity. Taken together, we provide evidence that ZKSCAN3, a zinc finger transcription factor, plays a critical role in promoting prostate cancer cell migration.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Chambers A. The metastatic process: basic research and clinical implications. Oncol Res. 1999;11:161–168. - PubMed
-
- Grimstad I. Direct evidence that cancer cell locomotion contributes importantly to invasion. Exp Cell Res. 1987;173:515–523. - PubMed
-
- Hendrix M. Editorial: potential clinical use of exploiting metastasis suppressor genes to regulate prostatic cancer. J Urol. 2003;169:1134. - PubMed
-
- Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute; Bethesda: 2011.
-
- Janer M, Friedrichsen D, Stanford J, Badzioch M, Kolb S, Deutsch K, et al. Genomic scan of 254 hereditary prostate cancer families. Prostate. 2003;57:309–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

