Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis
- PMID: 22531785
- PMCID: PMC3365430
- DOI: 10.1038/emboj.2012.93
Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis
Abstract
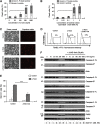
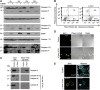
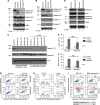
Bacterial pathogens modulate host cell apoptosis to establish a successful infection. Pore-forming toxins (PFTs) secreted by pathogenic bacteria are major virulence factors and have been shown to induce various forms of cell death in infected cells. Here we demonstrate that the highly conserved caspase-2 is required for PFT-mediated apoptosis. Despite being the second mammalian caspase to be identified, the role of caspase-2 during apoptosis remains enigmatic. We show that caspase-2 functions as an initiator caspase during Staphylococcus aureus α-toxin- and Aeromonas aerolysin-mediated apoptosis in epithelial cells. Downregulation of caspase-2 leads to a strong inhibition of PFT-mediated apoptosis. Activation of caspase-2 is PIDDosome-independent, and endogenous caspase-2 is recruited to a high-molecular-weight complex in α-toxin-treated cells. Interestingly, prevention of PFT-induced potassium efflux inhibits the formation of caspase-2 complex, leading to its inactivation, thus resisting apoptosis. These results revealed a thus far unknown, obligatory role for caspase-2 as an initiator caspase during PFT-mediated apoptosis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

