CD8+ T-cells expressing interferon gamma or perforin play antagonistic roles in heart injury in experimental Trypanosoma cruzi-elicited cardiomyopathy
- PMID: 22532799
- PMCID: PMC3330123
- DOI: 10.1371/journal.ppat.1002645
CD8+ T-cells expressing interferon gamma or perforin play antagonistic roles in heart injury in experimental Trypanosoma cruzi-elicited cardiomyopathy
Abstract
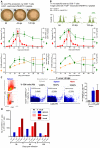
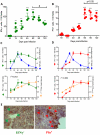
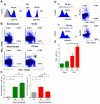
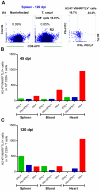
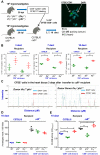
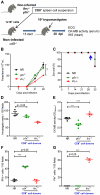
In Chagas disease, CD8(+) T-cells are critical for the control of Trypanosoma cruzi during acute infection. Conversely, CD8(+) T-cell accumulation in the myocardium during chronic infection may cause tissue injury leading to chronic chagasic cardiomyopathy (CCC). Here we explored the role of CD8(+) T-cells in T. cruzi-elicited heart injury in C57BL/6 mice infected with the Colombian strain. Cardiomyocyte lesion evaluated by creatine kinase-MB isoenzyme activity levels in the serum and electrical abnormalities revealed by electrocardiogram were not associated with the intensity of heart parasitism and myocarditis in the chronic infection. Further, there was no association between heart injury and systemic anti-T. cruzi CD8(+) T-cell capacity to produce interferon-gamma (IFNγ) and to perform specific cytotoxicity. Heart injury, however, paralleled accumulation of anti-T. cruzi cells in the cardiac tissue. In T. cruzi infection, most of the CD8(+) T-cells segregated into IFNγ(+) perforin (Pfn)(neg) or IFNγ(neg)Pfn(+) cell populations. Colonization of the cardiac tissue by anti-T. cruzi CD8(+)Pfn(+) cells paralleled the worsening of CCC. The adoptive cell transfer to T. cruzi-infected cd8(-/-) recipients showed that the CD8(+) cells from infected ifnγ(-/-)pfn(+/+) donors migrate towards the cardiac tissue to a greater extent and caused a more severe cardiomyocyte lesion than CD8(+) cells from ifnγ(+/+)pfn(-/-) donors. Moreover, the reconstitution of naïve cd8(-/-) mice with CD8(+) cells from naïve ifnγ(+/+)pfn(-/-) donors ameliorated T. cruzi-elicited heart injury paralleled IFNγ(+) cells accumulation, whereas reconstitution with CD8(+) cells from naïve ifnγ(-/-)pfn(+/+) donors led to an aggravation of the cardiomyocyte lesion, which was associated with the accumulation of Pfn(+) cells in the cardiac tissue. Our data support a possible antagonist effect of CD8(+)Pfn(+) and CD8(+)IFNγ(+) cells during CCC. CD8(+)IFNγ(+) cells may exert a beneficial role, whereas CD8(+)Pfn(+) may play a detrimental role in T. cruzi-elicited heart injury.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–13402. - PubMed
-
- Higuchi Mde L, Gutierrez PS, Aiello VD, Palomino S, Bocchi E, et al. Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: comparison with myocardial rejection process. Virchows Arch A Pathol Anat Histopathol. 1993;423:157–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

