Generation of retinal pigment epithelial cells from small molecules and OCT4 reprogrammed human induced pluripotent stem cells
- PMID: 22532929
- PMCID: PMC3328503
- DOI: 10.5966/sctm.2011-0057
Generation of retinal pigment epithelial cells from small molecules and OCT4 reprogrammed human induced pluripotent stem cells
Abstract
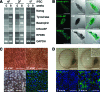
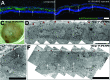
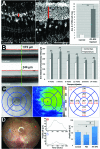
Autologous retinal pigment epithelium (RPE) grafts derived from induced pluripotent stem cells (iPSCs) may be used to cure blinding diseases in which RPE dysfunction results in photoreceptor degeneration. Four-, two-, and one-factor-derived iPSCs (4F-, 2F-, and 1F-iPSCs, respectively) were differentiated into fully functional cuboidal pigmented cells in polarized monolayers that express RPE-specific markers. 1F-iPSCs-RPE (1F-iPS-RPE) strongly resembles primary human fetal RPE (hfRPE) based on proteomic and untargeted metabolomic analyses, and using novel in vivo imaging technology coupled with electroretinography, we demonstrated that 1F-iPS-RPE mediate anatomical and functional rescue of photoreceptors after transplantation in an animal model of RPE-mediated retinal degeneration. 1F-iPS0RPE cells were injected subretinally as a suspension and formed a monolayer dispersed between host RPE cells. Furthermore, 1F-iPS-RPE do not simply provide trophic support to rescue photoreceptors as previously speculated but actually phagocytose photoreceptor outer segments in vivo and maintain visual cycling. Thus, 1f-iPS-RPE grafts may be superior to conventional iPS-RPE for clinical use because 1F-IPS-RPE closely resemble hfRPE, mediate anatomical and functional photoreceptor rescue in vivo, and are generated using a reduced number of potentially oncogenic reprogramming factors.
Keywords: Retinal pigment epithelium; differentiation; induced pluripotent stem cells; small molecules.
Figures


References
-
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Koch P, Kokaia Z, Lindvall O, et al. Emerging concepts in neural stem cell research: Autologous repair and cell-based disease modelling. Lancet Neurol. 2009;8:819–829. - PubMed
-
- Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. - PubMed
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

