The expression of the glucocorticoid receptor in human erythroblasts is uniquely regulated by KIT ligand: implications for stress erythropoiesis
- PMID: 22533504
- PMCID: PMC3623384
- DOI: 10.1089/scd.2011.0676
The expression of the glucocorticoid receptor in human erythroblasts is uniquely regulated by KIT ligand: implications for stress erythropoiesis
Abstract
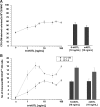
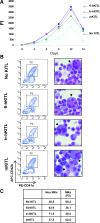
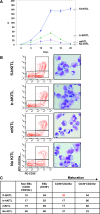
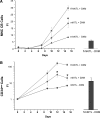
Studies in mice indicated that activation of the erythroid stress pathway requires the presence of both soluble KIT ligand (KITL) and the glucocorticoid receptor (GR). To clarify the relative role of KITL and GR in stress erythropoiesis in humans, the biological activities of soluble full length- (fl-, 26-190 aa), carboxy-terminus truncated (tr-, 26-162 aa) human (hKITL) and murine (mKITL) KITL in cultures of cord blood (CB) mononuclear cells (MNCs) and CD34(pos) cells that mimic either steady state (growth factors alone) or stress (growth factors plus dexamethasone [DXM]) erythropoeisis were investigated. In steady state cultures, the KITLs investigated were equally potent in sustaining growth of hematopoietic colonies and expansion of megakaryocytes (MK) and erythroid precursors (EBs). By contrast, under stress erythropoiesis conditions, fl-hKITL generated greater numbers of EBs (fold increase [FI]=140) than tr-hKITL or mKITL (FI=20-40). Flow cytometric analyses indicated that only EBs generated with fl-hKITL remained immature (>70% CD36(pos)/CD235a(neg/low)), and therefore capable to proliferate, until day 8-12 in response to DXM. Signaling studies indicated that all KITLs investigated induced EBs to phosphorylate signal transducer and activator of transcription 5 (STAT5) but that extracellular-signaling-regulated-kinases (ERK) activation was observed mainly in the presence of fl-hKITL. EBs exposed to fl-hKITL also expressed higher levels of GRα than those exposed to mKITL (and tr-hKITL) which were reduced upon exposure to the ERK inhibitor U0126. These data reveal a unique requirement for fl-hKITL in the upregulation of GRα and optimal EB expansion in cultures that mimic stress erythropoiesis.
Figures




Similar articles
-
The dominant negative β isoform of the glucocorticoid receptor is uniquely expressed in erythroid cells expanded from polycythemia vera patients.Blood. 2011 Jul 14;118(2):425-36. doi: 10.1182/blood-2010-07-296921. Epub 2011 Feb 25. Blood. 2011. PMID: 21355091 Free PMC article.
-
A KIT juxtamembrane PY567 -directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis.Exp Hematol. 2009 Feb;37(2):159-71. doi: 10.1016/j.exphem.2008.10.009. Epub 2008 Dec 18. Exp Hematol. 2009. PMID: 19100679 Free PMC article.
-
Stat5 signaling specifies basal versus stress erythropoietic responses through distinct binary and graded dynamic modalities.PLoS Biol. 2012 Aug;10(8):e1001383. doi: 10.1371/journal.pbio.1001383. Epub 2012 Aug 28. PLoS Biol. 2012. PMID: 22969412 Free PMC article.
-
Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells.Leukemia. 1996 Apr;10(4):588-99. Leukemia. 1996. PMID: 8618433 Review.
-
[HSP70, an erythropoiesis regulator that determines the fate of erythroblasts between death and differentiation].Transfus Clin Biol. 2013 May;20(2):144-7. doi: 10.1016/j.tracli.2013.03.005. Epub 2013 May 2. Transfus Clin Biol. 2013. PMID: 23643330 Review. French.
Cited by
-
Patients with hypercortisolemic Cushing disease possess a distinct class of hematopoietic progenitor cells leading to erythrocytosis.Haematologica. 2023 Apr 1;108(4):1053-1067. doi: 10.3324/haematol.2021.280542. Haematologica. 2023. PMID: 35861015 Free PMC article.
-
Concise Review: Advanced Cell Culture Models for Diamond Blackfan Anemia and Other Erythroid Disorders.Stem Cells. 2018 Feb;36(2):172-179. doi: 10.1002/stem.2735. Epub 2017 Dec 5. Stem Cells. 2018. PMID: 29124822 Free PMC article. Review.
-
β-thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies.Haematologica. 2015 Apr;100(4):418-30. doi: 10.3324/haematol.2014.114827. Haematologica. 2015. PMID: 25828088 Free PMC article. Review.
-
Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans.Nat Commun. 2016 Mar 21;7:10967. doi: 10.1038/ncomms10967. Nat Commun. 2016. PMID: 26997371 Free PMC article.
-
Childhood Adversity Is Associated With Increased KITLG Methylation in Healthy Individuals but Not in Bipolar Disorder Patients.Front Psychiatry. 2019 Jan 22;9:743. doi: 10.3389/fpsyt.2018.00743. eCollection 2018. Front Psychiatry. 2019. PMID: 30723428 Free PMC article.
References
-
- Papayannopoulou T. Migliaccio AR. Abkowitz JL. D'Andrea AD. Biology of erythropoiesis, erythroid differentiation, and maturation. In: Hoffman R, editor; Benz EJ, editor; Shattil S, editor. Hematology: Basic Principles and Practise. 5. Churchill Livingstone; New York: 2009. pp. 276–294.
-
- Besmer P. Kit-ligand-stem cell factor. In: Garland JM, editor; Quesenberry PJ, editor; Hilton DJ, editor. Colony-Stimulating Factors: Molecular and Cellular Biology. Marcel Dekker; New York: 1997. pp. 369–404.
-
- Migliaccio G. Migliaccio AR. Druzin ML. Giardina PJ. Zsebo KM. Adamson JW. Effects of recombinant human stem cell factor (SCF) on the growth of human progenitor cells in vitro. J Cell Physiol. 1991;148:503–509. - PubMed
-
- Migliaccio G. Migliaccio AR. Druzin ML. Giardina PJ. Zsebo KM. Adamson JW. Long-term generation of colony-forming cells in liquid culture of CD34+ cord blood cells in the presence of recombinant human stem cell factor. Blood. 1992;79:2620–2627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

