Disease progression model in subjects with mild cognitive impairment from the Alzheimer's disease neuroimaging initiative: CSF biomarkers predict population subtypes
- PMID: 22534009
- PMCID: PMC3555054
- DOI: 10.1111/j.1365-2125.2012.04308.x
Disease progression model in subjects with mild cognitive impairment from the Alzheimer's disease neuroimaging initiative: CSF biomarkers predict population subtypes
Abstract
Aim: The objective is to develop a semi-mechanistic disease progression model for mild cognitive impairment (MCI) subjects. The model aims to describe the longitudinal progression of ADAS-cog scores from the Alzheimer's disease neuroimaging initiative trial that had data from 198 MCI subjects with cerebrospinal fluid (CSF) information who were followed for 3 years.
Method: Various covariates were tested on disease progression parameters and these variables fell into six categories: imaging volumetrics, biochemical, genetic, demographic, cognitive tests and CSF biomarkers.
Results: CSF biomarkers were associated with both baseline disease score and disease progression rate in subjects with MCI. Baseline disease score was also correlated with atrophy measured using hippocampal volume. Progression rate was also predicted by executive functioning as measured by the Trail B-test.
Conclusion: CSF biomarkers have the ability to discriminate MCI subjects into sub-populations that exhibit markedly different rates of disease progression on the ADAS-cog scale. These biomarkers can therefore be utilized for designing clinical trials enriched with subjects that carry the underlying disease pathology.
© 2012 Janssen Pharmaceuticals, Inc. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
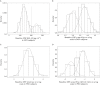
 , With disease pathology;
, With disease pathology;  , Without disease pathology
, Without disease pathology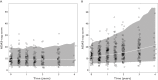
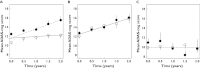
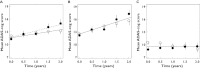
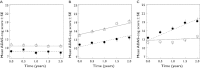
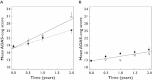
 log CSF p-tau181p : Aβ1-42 ratio ≤−1.86
log CSF p-tau181p : Aβ1-42 ratio ≤−1.86References
-
- Petersen RC. Mild cognitive impairment clinical trials. Nat Rev Drug Discov. 2003;2:646–53. - PubMed
-
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. International Psychogeriatric Association Expert Conference on mild cognitive impairment. Mild cognitive impairment. Lancet. 2006;367:1262–70. - PubMed
-
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ Alzheimer's Disease Neuroimaging Initiative. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–56. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

