Opposite regulation by PI3K/Akt and MAPK/ERK pathways of tissue factor expression, cell-associated procoagulant activity and invasiveness in MDA-MB-231 cells
- PMID: 22534171
- PMCID: PMC3394220
- DOI: 10.1186/1756-8722-5-16
Opposite regulation by PI3K/Akt and MAPK/ERK pathways of tissue factor expression, cell-associated procoagulant activity and invasiveness in MDA-MB-231 cells
Abstract
Background: Tissue factor (TF), an initiator of blood coagulation, participates in cancer progression and metastasis. We recently found that inhibition of MAPK/ERK upregulated both full length TF (flTF) and soluble isoform TF (asTF) gene expression and cell-associated TF activity in breast cancer MDA-MB-231 cells. We explored the possible mechanisms, especially the possible interaction with EGFR and PI3K/Akt pathways.
Methods: A plasmid containing TF promoter -2174 ~ +128 plus luciferase reporter gene was introduced into MDA-MB-231 cells to evaluate TF promoter activity. In order to study the interaction of these pathways, ERK inhibitor (PD98059), PI3K inhibitors (LY294002, wortmannin), Akt inhibitor (A6730), and EGFR inhibitor (erlotinib) as well as the corresponding siRNAs were used to treat MDA-MB-231 cells, and ovarian cancer OVCAR-3 and SKOV-3 cells. Quantitative PCR and western blot were used to determine TF expression. One stage clotting assays were used to measure pro-coagulation activity of the MDA-MB-231 cells.
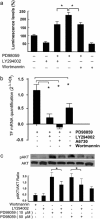
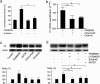
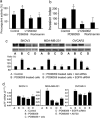
Results: We show that PI3K inhibitors LY294002, wortmannin and A6730 significantly inhibited TF promoter activity, and reduced TF mRNA and protein levels due to the inhibition of Akt phosphorylation. In contrast, ERK inhibitor PD98059 and ERK siRNA enhanced TF promoter activity by 2.5 fold and induced an increase in TF mRNA and protein levels in a dose dependent manner in these cells. The PI3K/Akt pathway was shown to be involved in PD98059-induced TF expression because the induction was inhibited by PI3K/Akt inhibitors. Most interestingly, the EGFR inhibitor erlotinib and EGFR siRNA also significantly suppressed PD98059- or ERK siRNA-induced TF promoter activity and TF protein expression. Similar results were found with ovarian cancer cells SKOV-3 and OVCAR-3. Furthermore, in MDA-MB-231, mRNA levels of asTF were regulated in a similar way to that of TF in response to the cell treatment.
Conclusions: This study showed a regulatory mechanism in which MAPK/ERK signals inhibit EGFR/PI3K/Akt-mediated TF expression in breast cancer MDA-MB-231 cells. The same regulation was observed in ovarian cancer OVCAR-3 and SKOV-3 cells. Interestingly, we observed that both flTF and asTF could be regulated in a parallel manner in MDA-MB-231. As the PI3K/Akt pathway and EGFR regulate TF expression in cancer cells, targeting these signaling components is expected to potentially inhibit TF expression-associated tumor progression.
Figures

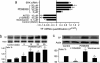
References
-
- Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–1550. doi: 10.1161/01.ATV.0000171155.05809.bf. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

