Meta-analysis of bleeding complications associated with cardiac rhythm device implantation
- PMID: 22534249
- PMCID: PMC3626087
- DOI: 10.1161/CIRCEP.111.969105
Meta-analysis of bleeding complications associated with cardiac rhythm device implantation
Abstract
Background: Many patients receiving cardiac rhythm devices have conditions requiring antiplatelet (AP) and/or anticoagulant (AC) therapy. Current guidelines recommend a heparin-bridging strategy (HBS) for anticoagulated patients with moderate/high risk for thrombosis. Several studies reported lower bleeding risk with continued oral anticoagulation rather than HBS. The best strategy for perioperative management of patients on AP therapy is less clear. The present study was designed as a meta-analysis of device implantation-associated bleeding complications using different AC/AP therapies.
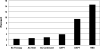
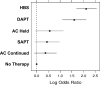
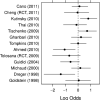
Methods and results: PubMed and Cochrane Database searches identified articles based on design, outcomes, and available data. Device recipients were grouped as follows: no therapy, aspirin only, AC held, AC continued, dual AP, and HBS. The primary outcome was defined as a bleeding complication including hematoma, transfusion, or prolonged hospital stay. Thirteen articles were identified for analysis including 5978 patients. The combined incidence of bleeding complications was 274 of 5978 (4.6%), ranging from 2.2% (no therapy) to 14.6% (HBS). The estimated odds of bleeding were increased by 8.3 (95% CI, 5.5-12.9) times in the HBS group, 5.0 (95% CI, 3.0-8.3) for dual AP therapy, 1.7 (95% CI, 1.0-3.1) for AC held, 1.6 (95% CI, 0.9-2.6) for AC continued, and 1.5 (95% CI, 0.9-2.3) for aspirin only relative to the no therapy group. HBS significantly increased bleeding events compared with holding or continuing AC. Continuing AC did not increase bleeding events compared with no therapy.
Conclusions: Continuing AC appears safer than HBS for device implantation. Dual AP therapy but not continuing AC carries a significant risk of bleeding.
Figures

References
-
- Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–62. - PubMed
-
- Jamula E, Douketis JD, Schulman S. Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide. J Thromb Haemost. 2008;6:1615–1621. - PubMed
-
- de Bono J, Nazir S, Ruparelia N, Bashir Y, Betts T, Rajappan K. Perioperative management of anticoagulation during device implantation-the UK perspective. Pacing Clin Electrophysiol. 2010;33:389–393. - PubMed
-
- Hammerstingl C, Omran H. Perioperative bridging of chronic oral anticoagulation in patients undergoing pacemaker implantation-a study in 200 patients. Europace. 2011;13:1304–1310. - PubMed
-
- Cheng A, Nazarian S, Brinker JA, Tompkins C, Spragg DD, Leng CT, Halperin H, Tandri H, Sinha SK, Marine JE, Calkins H, Tomaselli GF, Berger RD, Henrikson CA. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm. 2011;8:536–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

