Molecular mechanism of ATP binding and ion channel activation in P2X receptors
- PMID: 22535247
- PMCID: PMC3391165
- DOI: 10.1038/nature11010
Molecular mechanism of ATP binding and ion channel activation in P2X receptors
Abstract
P2X receptors are trimeric ATP-activated ion channels permeable to Na+, K+ and Ca2+. The seven P2X receptor subtypes are implicated in physiological processes that include modulation of synaptic transmission, contraction of smooth muscle, secretion of chemical transmitters and regulation of immune responses. Despite the importance of P2X receptors in cellular physiology, the three-dimensional composition of the ATP-binding site, the structural mechanism of ATP-dependent ion channel gating and the architecture of the open ion channel pore are unknown. Here we report the crystal structure of the zebrafish P2X4 receptor in complex with ATP and a new structure of the apo receptor. The agonist-bound structure reveals a previously unseen ATP-binding motif and an open ion channel pore. ATP binding induces cleft closure of the nucleotide-binding pocket, flexing of the lower body β-sheet and a radial expansion of the extracellular vestibule. The structural widening of the extracellular vestibule is directly coupled to the opening of the ion channel pore by way of an iris-like expansion of the transmembrane helices. The structural delineation of the ATP-binding site and the ion channel pore, together with the conformational changes associated with ion channel gating, will stimulate development of new pharmacological agents.
Conflict of interest statement
The authors declare no competing financial interests
Figures
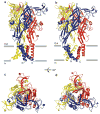
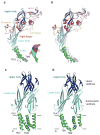


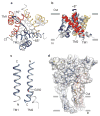
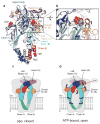
References
-
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. - PubMed
-
- Valera S, et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. - PubMed
-
- Webb TE, et al. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. - PubMed
-
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. - PubMed
-
- Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

