Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice
- PMID: 22535377
- PMCID: PMC3505865
- DOI: 10.1136/gutjnl-2011-301689
Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice
Abstract
Background: Obesity is associated with accumulation of macrophages in white adipose tissue (WAT), which contribute to the development of insulin resistance. Germ-free (GF) mice have reduced adiposity and are protected against diet-induced obesity,
Objective: To investigate whether the gut microbiota and, specifically, gut-derived lipopolysaccharide (LPS) promote WAT inflammation and contribute to impaired glucose metabolism.
Method: Macrophage composition and expression of proinflammatory and anti-inflammatory markers were compared in WAT of GF, conventionally raised and Escherichia coli-monocolonised mice. Additionally, glucose and insulin tolerance in these mice was determined.
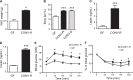
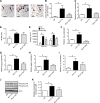
Results: The presence of a gut microbiota resulted in impaired glucose metabolism and increased macrophage accumulation and polarisation towards the proinflammatory M1 phenotype in WAT. Monocolonisation of GF mice for 4 weeks with E. coli W3110 or the isogenic strain MLK1067 (which expresses LPS with reduced immunogenicity) resulted in impaired glucose and insulin tolerance and promoted M1 polarisation of CD11b cells in WAT. However, colonisation with E. coli W3110 but not MLK1067 promoted macrophage accumulation and upregulation of proinflammatory and anti-inflammatory gene expression as well as JNK phosphorylation.
Conclusion: Gut microbiota induced LPS-dependent macrophage accumulation in WAT, whereas impairment of systemic glucose metabolism was not dependent on LPS. These results indicate that macrophage accumulation in WAT does not always correlate with impaired glucose metabolism.
Conflict of interest statement
Figures


Comment in
-
Microbes and metabolic health.Gut. 2012 Dec;61(12):1655-6. doi: 10.1136/gutjnl-2012-302507. Epub 2012 May 25. Gut. 2012. PMID: 22637700 No abstract available.
References
-
- Rabot S, Membrez M, Bruneau A, et al. Germ-free c57bl/6j mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010:4948–59 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
