Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug
- PMID: 22535622
- PMCID: PMC3888155
- DOI: 10.1093/jac/dks147
Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug
Abstract
Objectives: Infection with yellow fever virus (YFV), the prototypic mosquito-borne flavivirus, causes severe febrile disease with haemorrhage, multi-organ failure and a high mortality. Moreover, in recent years the Flavivirus genus has gained further attention due to re-emergence and increasing incidence of West Nile, dengue and Japanese encephalitis viruses. Potent and safe antivirals are urgently needed.
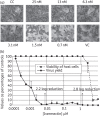
Methods: Starting from the crystal structure of the NS3 helicase from Kunjin virus (an Australian variant of West Nile virus), we identified a novel, unexploited protein site that might be involved in the helicase catalytic cycle and could thus in principle be targeted for enzyme inhibition. In silico docking of a library of small molecules allowed us to identify a few selected compounds with high predicted affinity for the new site. Their activity against helicases from several flaviviruses was confirmed in in vitro helicase/enzymatic assays. The effect on the in vitro replication of flaviviruses was then evaluated.
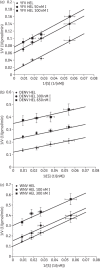
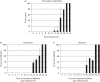
Results: Ivermectin, a broadly used anti-helminthic drug, proved to be a highly potent inhibitor of YFV replication (EC₅₀ values in the sub-nanomolar range). Moreover, ivermectin inhibited, although less efficiently, the replication of several other flaviviruses, i.e. dengue fever, Japanese encephalitis and tick-borne encephalitis viruses. Ivermectin exerts its effect at a timepoint that coincides with the onset of intracellular viral RNA synthesis, as expected for a molecule that specifically targets the viral helicase.
Conclusions: The well-tolerated drug ivermectin may hold great potential for treatment of YFV infections. Furthermore, structure-based optimization may result in analogues exerting potent activity against flaviviruses other than YFV.
Figures

References
-
- Gould EA, Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–9. doi:10.1016/S0140-6736(08)60238-X. - DOI - PubMed
-
- Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11–20. doi:10.1016/S1473-3099(01)00016-0. - DOI - PubMed
-
- Staples JE, Monath TP. Yellow fever: 100 years of discovery. JAMA. 2008;300:960–2. doi:10.1001/jama.300.8.960. - DOI - PubMed
-
- Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–29. doi:10.1146/annurev.ento.52.110405.091454. - DOI - PubMed
-
- Ellis BR, Barrett AD. The enigma of yellow fever in East Africa. Rev Med Virol. 2008;18:331–46. doi:10.1002/rmv.584. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

