Myeloma as a model for the process of metastasis: implications for therapy
- PMID: 22535658
- PMCID: PMC3390959
- DOI: 10.1182/blood-2012-01-379024
Myeloma as a model for the process of metastasis: implications for therapy
Abstract
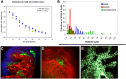
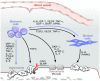
Multiple myeloma (MM) is a plasma cell dyscrasia characterized by the presence of multiple myelomatous "omas" throughout the skeleton, indicating that there is continuous trafficking of tumor cells to multiple areas in the bone marrow niches. MM may therefore represent one of the best models to study cell trafficking or cell metastasis. The process of cell metastasis is described as a multistep process, the invasion-metastasis cascade. This involves cell invasion, intravasation into nearby blood vessels, passage into the circulation, followed by homing into predetermined distant tissues, the formation of new foci of micrometastases, and finally the growth of micrometastasis into macroscopic tumors. This review discusses the significant advances that have been discovered in the complex process of invasion-metastasis in epithelial carcinomas and cell trafficking in hematopoietic stem cells and how this process relates to progression in MM. This progression is mediated by clonal intrinsic factors that mediate tumor invasiveness as well as factors present in the tumor microenvironment that are permissive to oncogenic proliferation. Therapeutic agents that target the different steps of cell dissemination and progression are discussed. Despite the significant advances in the treatment of MM, better therapeutic agents that target this metastatic cascade are urgently needed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

