Potential repurposing of known drugs as potent bacterial β-glucuronidase inhibitors
- PMID: 22535688
- PMCID: PMC8284931
- DOI: 10.1177/1087057112444927
Potential repurposing of known drugs as potent bacterial β-glucuronidase inhibitors
Abstract
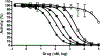
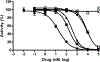
The active metabolite of the chemotherapeutic irinotecan, SN-38, is detoxified through glucuronidation and then excreted into the gastrointestinal tract. Intestinal bacteria convert the glucuronidated metabolite back to the toxic SN-38 using β-glucuronidase (GUS), resulting in debilitating diarrhea. Inhibiting GUS activity may relieve this side effect of irinotecan. In this study, we sought to determine whether any known drugs have GUS inhibitory activity. We screened a library of Food and Drug Administration-approved drugs with a cell-free biochemical enzyme assay using purified bacterial GUS. After triage, five drugs were confirmed to inhibit purified bacterial GUS. Three of these were the monoamine oxidase inhibitors nialamide, isocarboxazid, and phenelzine with average IC(50) values for inhibiting GUS of 71, 128, and 2300 nM, respectively. The tricyclic antidepressant amoxapine (IC(50) = 388 nM) and the antimalarial mefloquine (IC(50) = 1.2 µM) also had activity. Nialamide, isocarboxazid, and amoxapine had no significant activity against purified mammalian GUS but showed potent activity for inhibiting endogenous GUS activity in a cell-based assay using living intact Escherichia coli with average IC(50) values of 17, 336, and 119 nM, respectively. Thus, nialamide, isocarboxazid, and amoxapine have potential to be repurposed as therapeutics to reduce diarrhea associated with irinotecan chemotherapy and warrant further investigation for this use.
Figures
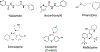
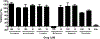
References
-
- Pommier Y (2006) Topoisomerase I inhibitors: camptothecins and beyond, Nat Rev Cancer 6, 789–802. - PubMed
-
- Pizzolato JF, and Saltz LB (2003) The camptothecins, Lancet 361, 2235–2242. - PubMed
-
- Smith NF, Figg WD, and Sparreboom A (2006) Pharmacogenetics of irinotecan metabolism and transport: an update, Toxicol In Vitro 20, 163–175. - PubMed
-
- Ma MK, and McLeod HL (2003) Lessons learned from the irinotecan metabolic pathway, Curr Med Chem 10, 41–49. - PubMed
-
- Mathijssen RHJ, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, and Sparreboom A (2001) Clinical Pharmacokinetics and Metabolism of Irinotecan (CPT-11), Clin Cancer Res 7, 2182–2194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

