Non-canonical translation in RNA viruses
- PMID: 22535777
- PMCID: PMC3542737
- DOI: 10.1099/vir.0.042499-0
Non-canonical translation in RNA viruses
Abstract
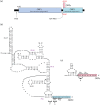
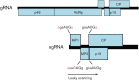
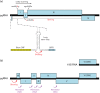
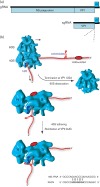
Viral protein synthesis is completely dependent upon the translational machinery of the host cell. However, many RNA virus transcripts have marked structural differences from cellular mRNAs that preclude canonical translation initiation, such as the absence of a 5' cap structure or the presence of highly structured 5'UTRs containing replication and/or packaging signals. Furthermore, whilst the great majority of cellular mRNAs are apparently monocistronic, RNA viruses must often express multiple proteins from their mRNAs. In addition, RNA viruses have very compact genomes and are under intense selective pressure to optimize usage of the available sequence space. Together, these features have driven the evolution of a plethora of non-canonical translational mechanisms in RNA viruses that help them to meet these challenges. Here, we review the mechanisms utilized by RNA viruses of eukaryotes, focusing on internal ribosome entry, leaky scanning, non-AUG initiation, ribosome shunting, reinitiation, ribosomal frameshifting and stop-codon readthrough. The review will highlight recently discovered examples of unusual translational strategies, besides revisiting some classical cases.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

