Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis
- PMID: 22535799
- PMCID: PMC3431140
- DOI: 10.1152/ajprenal.00110.2012
Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis
Abstract
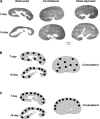
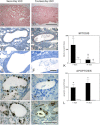
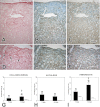
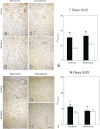
Unilateral ureteral obstruction (UUO) is the most widely used animal model of progressive renal disease. Although renal interstitial fibrosis is commonly used as an end point, recent studies reveal that obstructive injury to the glomerulotubular junction leads to the formation of atubular glomeruli. To quantitate the effects of UUO on the remainder of the nephron, renal tubular and interstitial responses were characterized in mice 7 and 14 days after UUO or sham operation under anesthesia. Fractional proximal tubular mass, cell proliferation, and cell death were measured by morphometry. Superoxide formation was identified by nitro blue tetrazolium, and oxidant injury was localized by 4-hydroxynonenol and 8-hydroxydeoxyguanosine. Fractional areas of renal vasculature, interstitial collagen, α-smooth muscle actin, and fibronectin were also measured. After 14 days of UUO, the obstructed kidney loses 19% of parenchymal mass, with a 65% reduction in proximal tubular mass. Superoxide formation is localized to proximal tubules, which undergo oxidant injury, apoptosis, necrosis, and autophagy, with widespread mitochondrial loss, resulting in tubular collapse. In contrast, mitosis and apoptosis increase in dilated collecting ducts, which remain patent through epithelial cell remodeling. Relative vascular volume fraction does not change, and interstitial matrix components do not exceed 15% of total volume fraction of the obstructed kidney. These unique proximal and distal nephron cellular responses reflect differential "fight-or-flight" responses to obstructive injury and provide earlier indexes of renal injury than do interstitial compartment responses. Therapies to prevent or retard progression of renal disease should include targeting proximal tubule injury as well as interstitial fibrosis.
Figures



References
-
- Buhrle CP, Hackenthal E, Helcher U, Lackner K, Nobiling R, Steinhausen M, Taugner R. Methods in laboratory investigation. The hydronephrotic kidney of the mouse as a tool for the intravital microscopy and in vitro electrophysiological studies of renin-containing cells. Lab Invest 54: 462–482, 1986 - PubMed
-
- Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, Chevalier RL. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int 63: 564–575, 2003 - PubMed
-
- Chevalier RL. Chronic partial ureteral obstruction in the neonatal guinea pig. II. Pressure gradients affecting glomerular filtration rate. Pediatr Res 18: 1271–1277, 1984 - PubMed
-
- Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

