Pexophagy: the selective degradation of peroxisomes
- PMID: 22536249
- PMCID: PMC3320016
- DOI: 10.1155/2012/512721
Pexophagy: the selective degradation of peroxisomes
Abstract
Peroxisomes are single-membrane-bounded organelles present in the majority of eukaryotic cells. Despite the existence of great diversity among different species, cell types, and under different environmental conditions, peroxisomes contain enzymes involved in β-oxidation of fatty acids and the generation, as well as detoxification, of hydrogen peroxide. The exigency of all eukaryotic cells to quickly adapt to different environmental factors requires the ability to precisely and efficiently control peroxisome number and functionality. Peroxisome homeostasis is achieved by the counterbalance between organelle biogenesis and degradation. The selective degradation of superfluous or damaged peroxisomes is facilitated by several tightly regulated pathways. The most prominent peroxisome degradation system uses components of the general autophagy core machinery and is therefore referred to as "pexophagy." In this paper we focus on recent developments in pexophagy and provide an overview of current knowledge and future challenges in the field. We compare different modes of pexophagy and mention shared and distinct features of pexophagy in yeast model systems, mammalian cells, and other organisms.
Figures

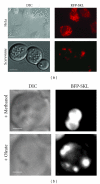



References
-
- Rhodin J. Correlation of ultrastructural organization and function in normal and experimentally changed proximal tubule cells of the mouse kidney. Stockholm, Sweden: Karolinska Institute; 1954. Ph.D. thesis.
-
- De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles) Physiological Reviews. 1966;46(2):323–357. - PubMed
-
- Hayashi M, Toriyama K, Kondo M, et al. Functional transformation of plant peroxisomes. Cell Biochemistry and Biophysics. 2000;32:295–304. - PubMed
-
- Michels PAM, Moyersoen J, Krazy H, Galland N, Herman M, Hannaert V. Peroxisomes, glyoxysomes and glycosomes. Molecular Membrane Biology. 2005;22(1-2):133–145. - PubMed
-
- Moyersoen J, Choe J, Fan E, Hol WGJ, Michels PAM. Biogenesis of peroxisomes and glycosomes: trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiology Reviews. 2004;28(5):603–643. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

