Impact of reporting bias in network meta-analysis of antidepressant placebo-controlled trials
- PMID: 22536359
- PMCID: PMC3335054
- DOI: 10.1371/journal.pone.0035219
Impact of reporting bias in network meta-analysis of antidepressant placebo-controlled trials
Abstract
Background: Indirect comparisons of competing treatments by network meta-analysis (NMA) are increasingly in use. Reporting bias has received little attention in this context. We aimed to assess the impact of such bias in NMAs.
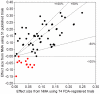
Methods: We used data from 74 FDA-registered placebo-controlled trials of 12 antidepressants and their 51 matching publications. For each dataset, NMA was used to estimate the effect sizes for 66 possible pair-wise comparisons of these drugs, the probabilities of being the best drug and ranking the drugs. To assess the impact of reporting bias, we compared the NMA results for the 51 published trials and those for the 74 FDA-registered trials. To assess how reporting bias affecting only one drug may affect the ranking of all drugs, we performed 12 different NMAs for hypothetical analysis. For each of these NMAs, we used published data for one drug and FDA data for the 11 other drugs.
Findings: Pair-wise effect sizes for drugs derived from the NMA of published data and those from the NMA of FDA data differed in absolute value by at least 100% in 30 of 66 pair-wise comparisons (45%). Depending on the dataset used, the top 3 agents differed, in composition and order. When reporting bias hypothetically affected only one drug, the affected drug ranked first in 5 of the 12 NMAs but second (n = 2), fourth (n = 1) or eighth (n = 2) in the NMA of the complete FDA network.
Conclusions: In this particular network, reporting bias biased NMA-based estimates of treatments efficacy and modified ranking. The reporting bias effect in NMAs may differ from that in classical meta-analyses in that reporting bias affecting only one drug may affect the ranking of all drugs.
Conflict of interest statement
Figures



References
-
- IOM (Institute of Medicine) Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009.
-
- Dickersin K. Health-care policy. To reform U.S. health care, start with systematic reviews. Science. 2010;329:516–517. - PubMed
-
- Volpp KG, Das A. Comparative effectiveness–thinking beyond medication A versus medication B. N Engl J Med. 2009;361:331–333. - PubMed
-
- Hochman M, McCormick D. Characteristics of published comparative effectiveness studies of medications. JAMA. 2010;303:951–958. - PubMed
-
- Lathyris DN, Patsopoulos NA, Salanti G, Ioannidis JP. Industry sponsorship and selection of comparators in randomized clinical trials. Eur J Clin Invest. 2010;40:172–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

