Characterisation of innate fungal recognition in the lung
- PMID: 22536422
- PMCID: PMC3334970
- DOI: 10.1371/journal.pone.0035675
Characterisation of innate fungal recognition in the lung
Abstract
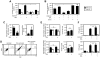
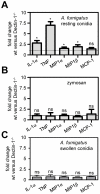
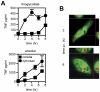
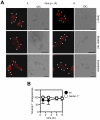
The innate recognition of fungi by leukocytes is mediated by pattern recognition receptors (PRR), such as Dectin-1, and is thought to occur at the cell surface triggering intracellular signalling cascades which lead to the induction of protective host responses. In the lung, this recognition is aided by surfactant which also serves to maintain the balance between inflammation and pulmonary function, although the underlying mechanisms are unknown. Here we have explored pulmonary innate recognition of a variety of fungal particles, including zymosan, Candida albicans and Aspergillus fumigatus, and demonstrate that opsonisation with surfactant components can limit inflammation by reducing host-cell fungal interactions. However, we found that this opsonisation does not contribute directly to innate fungal recognition and that this process is mediated through non-opsonic PRRs, including Dectin-1. Moreover, we found that pulmonary inflammatory responses to resting Aspergillus conidia were initiated by these PRRs in acidified phagolysosomes, following the uptake of fungal particles by leukocytes. Our data therefore provides crucial new insights into the mechanisms by which surfactant can maintain pulmonary function in the face of microbial challenge, and defines the phagolysosome as a novel intracellular compartment involved in the innate sensing of extracellular pathogens in the lung.
Conflict of interest statement
Figures



References
-
- Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 2011 - PubMed
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. - PubMed
-
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

