Evolution of transmitted HIV-1 drug resistance in HIV-1-infected patients in Italy from 2000 to 2010
- PMID: 22536753
- PMCID: PMC6029625
- DOI: 10.1111/j.1469-0691.2012.03847.x
Evolution of transmitted HIV-1 drug resistance in HIV-1-infected patients in Italy from 2000 to 2010
Abstract
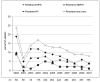
Prevalence and predictors of transmitted drug resistance (TDR), defined as the presence of at least one WHO surveillance drug resistance mutation (SDRM), were investigated in antiretroviral-naïve HIV-1-infected patients, with a genotypic resistance test (GRT) performed ≤6 months before starting cART between 2000 and 2010. 3163 HIV-1 sequences were selected (69% subtype B). Overall, the prevalence of TDR was 12% (13.2% subtype B, 9% non-B). TDR significantly declined overall and for the single drug classes. Older age independently predicted increased odds of TDR, whereas a more recent GRT, a higher HIV-RNA and C vs. B subtype predicted lower odds of TDR.
© 2012 The Authors. Clinical Microbiology and Infection © 2012 European Society of Clinical Microbiology and Infectious Diseases.
Figures



References
-
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008 Aug 23;372(9639):646–55. - PubMed
-
- Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008 Jul 31;22(12):1389–97. - PubMed
-
- Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009 Sep 5;374(9692):796–806. Epub 2009 Aug 3. Erratum in: Lancet. 2009 Dec 19-2010 Jan 1; 374(9707):2054. Lancet. 2009 Sep 5;374(9692):786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

