Management of patients coinfected with HCV and HIV: a close look at the role for direct-acting antivirals
- PMID: 22537439
- PMCID: PMC3637982
- DOI: 10.1053/j.gastro.2012.02.012
Management of patients coinfected with HCV and HIV: a close look at the role for direct-acting antivirals
Abstract
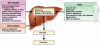
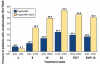
With the development of effective therapies against human immunodeficiency virus (HIV), hepatitis C virus (HCV) infection has become a major cause of morbidity and mortality among patients with both infections (coinfection). In addition to the high prevalence of chronic HCV, particularly among HIV-infected injection drug users, the rate of incident HIV infections is increasing among HIV-infected men who have sex with men, leading to recommendations for education and screening for HCV in this population. Liver disease is the second leading and, in some cases, a preventable cause of death among coinfected patients. Those at risk for liver disease progression are usually treated with a combination of interferon (IFN) and ribavirin (RBV), which is not highly effective; it has low rates of sustained virologic response (SVR), especially for coinfected patients with HCV genotype 1 and those of African descent. Direct-acting antivirals might overcome factors such as immunodeficiency that can reduce the efficacy of IFN. However, for now it remains challenging to treat coinfected patients due to interactions among drugs, additive drug toxicities, and the continued need for combination therapies that include pegylated IFN. Recently developed HCV protease inhibitors such as telaprevir and boceprevir, given in combination with pegylated IFN and RBV, could increase the rate of SVR with manageable toxicity and drug interactions. We review the latest developments and obstacles to treating coinfected patients.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. - PubMed
-
- Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. - PubMed
-
- Weber R, Sabin C, Friis-Møller N, et al. Liver-related deaths in persons infected with the HIV: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. - PubMed
-
- Naggie S, Park L, Gellad Z, et al. Healthcare utilization and mortality associated with chronic HIV and HCV: more to the story than chronic infection. Presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 27 to March 2, 2011; Abstract 915.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

