Intrinsic fibrillation of fast-acting insulin analogs
- PMID: 22538135
- PMCID: PMC3380767
- DOI: 10.1177/193229681200600209
Intrinsic fibrillation of fast-acting insulin analogs
Abstract
Background: Aggregation of insulin into insoluble fibrils (fibrillation) may lead to complications for diabetes patients such as reduced insulin potency, occlusion of insulin delivery devices, or potentially increased immunological potential. Even after extensive investigation of fibril formation in regular human insulin, there are little published data about the intrinsic fibrillation of fast-acting analogs. This article investigates and compares the intrinsic fibrillation of three fast-acting insulin analogs--lispro, aspart, and glulisine--as a function of their primary protein structure and exclusive of the stabilizing excipients that are added to their respective commercial formulations.
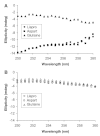
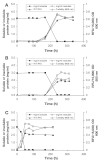
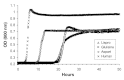
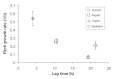
Methods: The insulin analogs underwent a buffer exchange into phosphate-buffered saline to remove formulation excipients and then were heated and agitated to characterize intrinsic fibrillation potentials devoid of excipient stabilizing effects. Different analytical methods were used to determine the amount of intrinsic fibrillation for the analogs. After initial lag times, intrinsic fibrillation was detected by an amyloid-specific stain. Precipitation of insulin was confirmed by ultraviolet analysis of soluble insulin and gravimetric measurement of insoluble insulin. Electron microscopy showed dense fibrous material, with individual fibrils that are shorter than typical insulin fibrils. Higher resolution kinetic analyses were carried out in 96-well plates to provide more accurate measures of lag times and fibril growth rates.
Results: All three analogs exhibited longer lag times and slower intrinsic fibrillation rates than human insulin, with glulisine and lispro rates slower than aspart. This is the first study comparing the intrinsic fibrillation of fast-acting insulin analogs without the stabilizing excipients found in their commercial formulations.
Conclusions: Data show different intrinsic fibrillation potentials based on primary molecular structures when the formulation excipients that are critical for stability are absent. Understanding intrinsic fibrillation potential is critical for evaluating insulin analog stability and device compatibility.
© 2012 Diabetes Technology Society.
Figures


References
-
- Mahler HC, Friess W, Grauschopf U, Kiese S. Protein aggregation: pathways, induction factors and analysis. J Pharm Sci. 2009;98(9):2909–2934. - PubMed
-
- Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–575. - PubMed
-
- Weiss WF, 4th, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98(4):1246–1277. - PubMed
-
- Patel J, Kothari R, Tunga R, Ritter NM, Tunga BS. Stability considerations for biopharmaceuticals, part 1. BioProc Int. 2011;9:20–31.
-
- Morozova-Roche L, Malisauskas M. A false paradise - mixed blessings in the protein universe: the amyloid as a new challenge in drug development. Curr Med Chem. 2007;14(11):1221–1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

