Protein polymer hydrogels by in situ, rapid and reversible self-gelation
- PMID: 22538198
- PMCID: PMC3801208
- DOI: 10.1016/j.biomaterials.2012.03.083
Protein polymer hydrogels by in situ, rapid and reversible self-gelation
Abstract
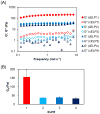
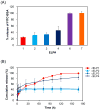
Protein-based biomaterials are an important class of materials for applications in biotechnology and medicine. The exquisite control of their composition, stereochemistry, and chain length offers unique opportunities to engineer biofunctionality, biocompatibility, and biodegradability into these materials. Here, we report the synthesis of a thermally responsive peptide polymer-based hydrogel composed of a recombinant elastin-like polypeptide (ELP) that rapidly forms a reversibly cross-linked hydrogel by the formation of intermolecular disulfide cross-links. To do so, we designed and synthesized ELPs that incorporate periodic cysteine residues (cELPs), and show that cELPs are thermally responsive protein polymers that display rapid gelation under physiologically relevant, mild oxidative conditions. Gelation of cELPs, at concentrations as low as 2.5 wt%, occurs in ≈ 2.5 min upon addition a low concentration of hydrogen peroxide (0.3 wt%). We show the utility of these hydrogels for the sustained release of a model protein in vitro, and demonstrate the ability of this injectable biomaterial to pervade tumors to maximize tumor coverage and retention time upon intratumoral injection. cELPs represent a new class of injectable reversibly cross-linked hydrogels with properties intermediate between ELP coacervates and chemically cross-linked ELP hydrogels that will find useful applications in drug delivery and tissue engineering.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






References
-
- Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80(1–3):9–28. - PubMed
-
- Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58(2):445–455. - PubMed
-
- Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):263–273. - PubMed
-
- Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–1481. - PubMed
-
- Nguyen MK, Lee DS. Injectable biodegradable hydrogels. Macromol Biosci. 2010;10(6):563–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

