Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis
- PMID: 22538462
- PMCID: PMC3702272
- DOI: 10.1159/000338669
Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis
Abstract
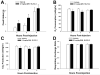
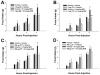
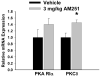
We examined the receptor subtypes and signal transduction mechanisms contributing to the estrogenic modulation of cannabinoid-induced changes in energy balance. Food intake and, in some cases, O2 consumption, CO2 production and the respiratory exchange ratio were evaluated in ovariectomized female guinea pigs treated s.c. with the cannabinoid receptor agonist WIN 55,212-2 or its cremephor/ethanol/0.9% saline vehicle, and either with estradiol benzoate (EB), the estrogen receptor (ER) α agonist PPT, the ERβ agonist DPN, the Gq-coupled membrane ER agonist STX, the GPR30 agonist G-1 or their respective vehicles. Patch-clamp recordings were performed in hypothalamic slices. EB, STX, PPT and G-1 decreased daily food intake. Of these, EB, STX and PPT blocked the WIN 55,212-2-induced increase in food intake within 1-4 h. The estrogenic diminution of cannabinoid-induced hyperphagia correlated with a rapid (within 15 min) attenuation of cannabinoid-mediated decreases in glutamatergic synaptic input onto arcuate neurons, which was completely blocked by inhibition of protein kinase C (PKC) and attenuated by inhibition of protein kinase A (PKA). STX, but not PPT, mimicked this rapid estrogenic effect. However, PPT abolished the cannabinoid-induced inhibition of glutamatergic neurotransmission in cells from animals treated 24 h prior. The estrogenic antagonism of this presynaptic inhibition was observed in anorexigenic proopiomelanocortin neurons. These data reveal that estrogens negatively modulate cannabinoid-induced changes in energy balance via Gq-coupled membrane ER- and ERα-mediated mechanisms involving activation of PKC and PKA. As such, they further our understanding of the pathways through which estrogens act to temper cannabinoid sensitivity in regulating energy homeostasis in females.
Copyright © 2012 S. Karger AG, Basel.
Figures

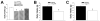

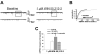



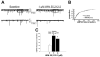
Similar articles
-
Role of neuronal nitric oxide synthase in the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis.J Neurophysiol. 2015 Feb 1;113(3):904-14. doi: 10.1152/jn.00615.2014. Epub 2014 Nov 12. J Neurophysiol. 2015. PMID: 25392169 Free PMC article.
-
Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis.Eur J Pharmacol. 2009 Nov 10;622(1-3):15-24. doi: 10.1016/j.ejphar.2009.09.001. Epub 2009 Sep 14. Eur J Pharmacol. 2009. PMID: 19758570 Free PMC article.
-
Cannabinoid-induced hyperphagia: correlation with inhibition of proopiomelanocortin neurons?Physiol Behav. 2007 Oct 22;92(3):507-19. doi: 10.1016/j.physbeh.2007.04.028. Epub 2007 Apr 29. Physiol Behav. 2007. PMID: 17532014 Free PMC article.
-
Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis.J Steroid Biochem Mol Biol. 2016 Jun;160:15-26. doi: 10.1016/j.jsbmb.2015.07.014. Epub 2015 Jul 29. J Steroid Biochem Mol Biol. 2016. PMID: 26232394 Free PMC article. Review.
-
Sex differences in the cannabinoid regulation of energy homeostasis.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1(0 1):S237-46. doi: 10.1016/j.psyneuen.2009.04.007. Psychoneuroendocrinology. 2009. PMID: 19427130 Free PMC article. Review.
Cited by
-
The role of AMP-activated protein kinase in the androgenic potentiation of cannabinoid-induced changes in energy homeostasis.Am J Physiol Endocrinol Metab. 2015 Mar 15;308(6):E482-95. doi: 10.1152/ajpendo.00421.2014. Epub 2014 Dec 30. Am J Physiol Endocrinol Metab. 2015. PMID: 25550281 Free PMC article.
-
Endocannabinoid Signaling at Hypothalamic Steroidogenic Factor-1/Proopiomelanocortin Synapses Is Sex- and Diet-Sensitive.Front Mol Neurosci. 2018 Jun 19;11:214. doi: 10.3389/fnmol.2018.00214. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29973869 Free PMC article.
-
Role of neuronal nitric oxide synthase in the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis.J Neurophysiol. 2015 Feb 1;113(3):904-14. doi: 10.1152/jn.00615.2014. Epub 2014 Nov 12. J Neurophysiol. 2015. PMID: 25392169 Free PMC article.
-
The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol.Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E632-40. doi: 10.1152/ajpendo.00281.2013. Epub 2013 Jul 2. Am J Physiol Endocrinol Metab. 2013. PMID: 23820624 Free PMC article.
-
Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective.Neuropsychopharmacology. 2018 Jan;43(1):34-51. doi: 10.1038/npp.2017.140. Epub 2017 Jul 17. Neuropsychopharmacology. 2018. PMID: 28811670 Free PMC article. Review.
References
-
- Matsuoka H, Tsubaki M, Yamazoe Y, Ogaki M, Satou T, Itoh T, Kusunoki T, Nishida S. Tamoxifen inhibits tumor cell invasion and metastasis in mouse melanoma through suppression of PKC/MEK/ERK and PKC/PI3K/Akt pathways. Exp Cell Res. 2009;315:2022–2032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

