Infection regulates pro-resolving mediators that lower antibiotic requirements
- PMID: 22538616
- PMCID: PMC3340015
- DOI: 10.1038/nature11042
Infection regulates pro-resolving mediators that lower antibiotic requirements
Abstract
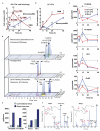
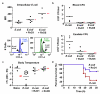
Underlying mechanisms for how bacterial infections contribute to active resolution of acute inflammation are unknown. Here, we performed exudate leukocyte trafficking and mediator-metabololipidomics of murine peritoneal Escherichia coli infections with temporal identification of pro-inflammatory (prostaglandins and leukotrienes) and specialized pro-resolving mediators (SPMs). In self-resolving E. coli exudates (10(5) colony forming units, c.f.u.), the dominant SPMs identified were resolvin (Rv) D5 and protectin D1 (PD1), which at 12 h were at significantly greater levels than in exudates from higher titre E. coli (10(7) c.f.u.)-challenged mice. Germ-free mice had endogenous RvD1 and PD1 levels higher than in conventional mice. RvD1 and RvD5 (nanograms per mouse) each reduced bacterial titres in blood and exudates, E. coli-induced hypothermia and increased survival, demonstrating the first actions of RvD5. With human polymorphonuclear neutrophils and macrophages, RvD1, RvD5 and PD1 each directly enhanced phagocytosis of E. coli, and RvD5 counter-regulated a panel of pro-inflammatory genes, including NF-κB and TNF-α. RvD5 activated the RvD1 receptor, GPR32, to enhance phagocytosis. With self-limited E. coli infections, RvD1 and the antibiotic ciprofloxacin accelerated resolution, each shortening resolution intervals (R(i)). Host-directed RvD1 actions enhanced ciprofloxacin's therapeutic actions. In 10(7) c.f.u. E. coli infections, SPMs (RvD1, RvD5, PD1) together with ciprofloxacin also heightened host antimicrobial responses. In skin infections, SPMs enhanced vancomycin clearance of Staphylococcus aureus. These results demonstrate that specific SPMs are temporally and differentially regulated during infections and that they are anti-phlogistic, enhance containment and lower antibiotic requirements for bacterial clearance.
Figures


Comment in
-
Infectious disease: Pro-resolving lipids offer a helping hand to antibiotics.Nat Rev Drug Discov. 2012 May 18;11(6):441. doi: 10.1038/nrd3760. Nat Rev Drug Discov. 2012. PMID: 22596252 No abstract available.
-
Infectious disease: Pro-resolving lipids offer a helping hand to antibiotics.Nat Rev Immunol. 2012 May 25;12(6):398-9. doi: 10.1038/nri3234. Nat Rev Immunol. 2012. PMID: 22627851 No abstract available.
References
-
- Houck JC, editor. Chemical Messengers of the Inflammatory Process. Elsevier/North-Holland Biomedical Press; 1979.
-
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. - PubMed
-
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. - PubMed
-
- Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. - PubMed
-
- Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011;50:35–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

