MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart
- PMID: 22538858
- PMCID: PMC3636396
- DOI: 10.1007/s11357-012-9407-9
MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart
Abstract
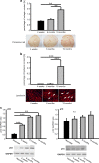
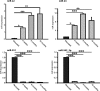
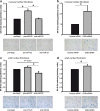
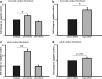
MicroRNAs (miRs) are small non- coding RNA molecules controlling a plethora of biological processes such as development, cellular survival and senescence. We here determined miRs differentially regulated during cardiac postnatal development and aging. Cardiac function, morphology and miR expression profiles were determined in neonatal, 4 weeks, 6 months and 19 months old normotensive male healthy C57/Bl6N mice. MiR-22 was most prominently upregulated during cardiac aging. Cardiac expression of its bioinformatically predicted target mimecan (osteoglycin, OGN) was gradually decreased with advanced age. Luciferase reporter assays validated mimecan as a bona fide miR-22 target. Both, miR-22 and its target mimecan were co- expressed in cardiac fibroblasts and smooth muscle cells. Functionally, miR-22 overexpression induced cellular senescence and promoted migratory activity of cardiac fibroblasts. Small interference RNA-mediated silencing of mimecan in cardiac fibroblasts mimicked the miR-22-mediated effects. Rescue experiments revealed that the effects of miR-22 on cardiac fibroblasts were only partially mediated by mimecan. In conclusion, miR-22 upregulation in the aging heart contributed at least partly to accelerated cardiac fibroblast senescence and increased migratory activity. Our results suggest an involvement of miR-22 in age-associated cardiac changes, such as cardiac fibrosis.
Figures


References
-
- Bentz H, Nathan RM, Rosen DM, Armstrong RM, Thompson AY, Segarini PR, Mathews MC, Dasch JR, Piez KA, Seyedin SM. Purification and characterization of a unique osteoinductive factor from bovine bone. J Biol Chem. 1989;264(34):20805–20810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
