Improvement of intertrochanteric bone quality in osteoporotic female rats after injection of polylactic acid-polyglycolic acid copolymer/collagen type I microspheres combined with bone mesenchymal stem cells
- PMID: 22539160
- PMCID: PMC3460074
- DOI: 10.1007/s00264-012-1543-4
Improvement of intertrochanteric bone quality in osteoporotic female rats after injection of polylactic acid-polyglycolic acid copolymer/collagen type I microspheres combined with bone mesenchymal stem cells
Abstract
Purpose: Osteoporosis mainly involves cancellous bone, and the spine and hip, with their relatively high cancellous bone to cortical bone ratio, are severely affected. Studies of bone mesenchymal stem cells (BMSCs) from osteoporotic patients and animal models have revealed that osteoporosis is often associated with reduction of BMSCs' proliferation and osteogenic differentiation. Our aim was to test whether polylactic acid-polyglycolic acid copolymer(PLGA)/collagen type I(CoI) microspheres combined with BMSCs could be used as injectable scaffolds to improve bone quality in osteoporotic female rats.
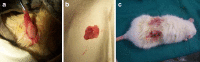
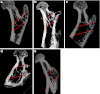
Methods: PLGA microspheres were coated with CoI. BMSCs of the third passage and were cultured with PLGA/CoI microspheres for seven days. Forty three-month-old female non-pregnant SD rats were ovariectomized to establish osteoporotic animal models. Three months after being ovariectomized, the osteoporotic rats were randomly divided into five groups: SHAM group, PBS group, cell group, microsphere (MS) group, and cell+MS group. Varying materials were injected into the intertrochanters of each group's rats. Twenty rats were sacrificed at one month and three months post-op, respectively. The femora were harvested in order to measure the intertrochanteric bone mineral density (BMD) with DEXA and trabecular thickness (Tb.Th), percentage of trabecular area (%Tb.Ar), bone volume fraction (BV/TV) and trabecular spacing (Tb.Sp) with Micro CT. One-way ANOVA and Kruskal-Wallis tests were used.
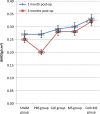
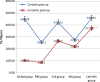
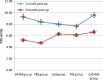
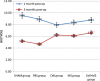
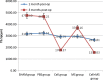
Results: BMSCs seeded on PLGA/CoI microspheres had a nice adhesion and proliferation. At one month post-op, the BMD (0.33 ± 0.01 g/cm(2)), Tb.Th (459.65 ± 28.31 μm), %Tb.Ar (9.61 ± 0.29 %) and Tb.Sp (2645.81 ± 94.91 μm) of the cell+ MS group were better than those of the SHAM group and the cell group. At three months post-op, the BMD (0.32 ± 0.01 g/cm(2)), Tb.Th (372.81 ± 38.45 μm), %Tb.Ar (6.65 ± 0.25 %), BV/TV (6.62 ± 0.25 %) and Tb.Sp (1559.03 ± 57.06 μm) of the cell + MS group were also better than those of the SHAM group and the cell group.
Conclusion: The PLGA/CoI microspheres combined with BMSCs can repair bone defects more quickly. This means that PLGA/CoI microspheres combined with BMSCs can promote trabecular reconstruction and improve bone quality in osteoporotic rats. This scaffold can provide a promising minimally invasive surgical tool for enhancement of bone fracture healing or prevention of fracture occurrence which will in turn minimize complications endemic to patients with osteoporosis.
Figures



References
-
- Chiu R, Smith KE, Ma GK, et al. Polymethylmethacrylate particles impair osteoprogenitor viability and expression of osteogenic transcription factors Runx2, osterix, and Dlx5. J Orthop Res. 2010;28(5):571–577. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

