DeltaPhage--a novel helper phage for high-valence pIX phagemid display
- PMID: 22539265
- PMCID: PMC3439877
- DOI: 10.1093/nar/gks341
DeltaPhage--a novel helper phage for high-valence pIX phagemid display
Abstract
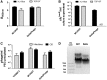
Phage display has been instrumental in discovery of novel binding peptides and folded domains for the past two decades. We recently reported a novel pIX phagemid display system that is characterized by a strong preference for phagemid packaging combined with low display levels, two key features that support highly efficient affinity selection. However, high diversity in selected repertoires are intimately coupled to high display levels during initial selection rounds. To incorporate this additional feature into the pIX display system, we have developed a novel helper phage termed DeltaPhage that allows for high-valence display on pIX. This was obtained by inserting two amber mutations close to the pIX start codon, but after the pVII translational stop, conditionally inactivating the helper phage encoded pIX. Until now, the general notion has been that display on pIX is dependent on wild-type complementation, making high-valence display unachievable. However, we found that DeltaPhage does facilitate high-valence pIX display when used with a non-suppressor host. Here, we report a side-by-side comparison with pIII display, and we find that this novel helper phage complements existing pIX phagemid display systems to allow both low and high-valence display, making pIX display a complete and efficient alternative to existing pIII phagemid display systems.
Figures

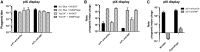


Similar articles
-
Expanding the versatility of phage display II: improved affinity selection of folded domains on protein VII and IX of the filamentous phage.PLoS One. 2011 Feb 24;6(2):e17433. doi: 10.1371/journal.pone.0017433. PLoS One. 2011. PMID: 21390283 Free PMC article.
-
Multivalent pIX phage display selects for distinct and improved antibody properties.Sci Rep. 2016 Dec 14;6:39066. doi: 10.1038/srep39066. Sci Rep. 2016. PMID: 27966617 Free PMC article.
-
Adapter-directed display: a modular design for shuttling display on phage surfaces.J Mol Biol. 2010 Feb 5;395(5):1088-101. doi: 10.1016/j.jmb.2009.11.068. Epub 2009 Dec 4. J Mol Biol. 2010. PMID: 19969002
-
Next generation phage display by use of pVII and pIX as display scaffolds.Methods. 2012 Sep;58(1):40-6. doi: 10.1016/j.ymeth.2012.07.005. Epub 2012 Jul 20. Methods. 2012. PMID: 22819858 Review.
-
The pComb3 Phagemid Family of Phage Display Vectors.Cold Spring Harb Protoc. 2024 Aug 1;2024(8):pdb.over107756. doi: 10.1101/pdb.over107756. Cold Spring Harb Protoc. 2024. PMID: 37460147 Review.
Cited by
-
Chimeric antigen receptor preparation from hybridoma to T-cell expression.Antib Ther. 2019 May 31;2(2):56-63. doi: 10.1093/abt/tbz007. eCollection 2019 Apr. Antib Ther. 2019. PMID: 33928223 Free PMC article.
-
Affinity maturation of TCR-like antibodies using phage display guided by structural modeling.Protein Eng Des Sel. 2022 Feb 17;35:gzac005. doi: 10.1093/protein/gzac005. Protein Eng Des Sel. 2022. PMID: 35871543 Free PMC article.
-
Oligopeptide m13 phage display in pathogen research.Viruses. 2013 Oct 16;5(10):2531-45. doi: 10.3390/v5102531. Viruses. 2013. PMID: 24136040 Free PMC article. Review.
-
Identification of a Phage Display-Derived Peptide Interacting with the N-Terminal Region of Factor VII Activating Protease (FSAP) Enables Characterization of Zymogen Activation.ACS Chem Biol. 2022 Sep 16;17(9):2631-2642. doi: 10.1021/acschembio.2c00538. Epub 2022 Sep 7. ACS Chem Biol. 2022. PMID: 36070465 Free PMC article.
-
Design and Characterization of a New pVII Combinatorial Phage Display Peptide Library for Protease Substrate Mining Using Factor VII Activating Protease (FSAP) as Model.Chembiochem. 2020 Jul 1;21(13):1875-1884. doi: 10.1002/cbic.201900705. Epub 2020 Apr 14. Chembiochem. 2020. PMID: 32180321 Free PMC article.
References
-
- Rakonjac J, Bennett NJ, Spagnuolo J, Gagic D, Russel M. Filamentous bacteriophage: biology, phage display and nanotechnology applications. Curr. Issues Mol. Biol. 2011;13:51–76. - PubMed
-
- Bradbury AR, Marks JD. Antibodies from phage antibody libraries. J. Immunol. Methods. 2004;290:29–49. - PubMed
-
- Dubel S, Stoevesandt O, Taussig MJ, Hust M. Generating recombinant antibodies to the complete human proteome. Trends Biotechnol. 2010;28:333–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

