Highly potent inhibitors of proprotein convertase furin as potential drugs for treatment of infectious diseases
- PMID: 22539349
- PMCID: PMC3381159
- DOI: 10.1074/jbc.M111.332643
Highly potent inhibitors of proprotein convertase furin as potential drugs for treatment of infectious diseases
Abstract
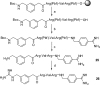
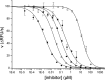
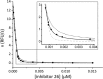
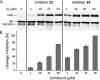
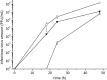
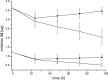
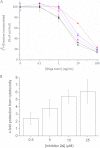
Optimization of our previously described peptidomimetic furin inhibitors was performed and yielded several analogs with a significantly improved activity. The most potent compounds containing an N-terminal 4- or 3-(guanidinomethyl)phenylacetyl residue inhibit furin with K(i) values of 16 and 8 pM, respectively. These analogs inhibit other proprotein convertases, such as PC1/3, PC4, PACE4, and PC5/6, with similar potency, whereas PC2, PC7, and trypsin-like serine proteases are poorly affected. Incubation of selected compounds with Madin-Darby canine kidney cells over a period of 96 h revealed that they exhibit great stability, making them suitable candidates for further studies in cell culture. Two of the most potent derivatives were used to inhibit the hemagglutinin cleavage and viral propagation of a highly pathogenic avian H7N1 influenza virus strain. The treatment with inhibitor 24 (4-(guanidinomethyl)phenylacetyl-Arg-Val-Arg-4-amidinobenzylamide) resulted in significantly delayed virus propagation compared with an inhibitor-free control. The same analog was also effective in inhibiting Shiga toxin activation in HEp-2 cells. This antiviral effect, as well as the protective effect against a bacterial toxin, suggests that inhibitors of furin or furin-like proprotein convertases could represent promising lead structures for future drug development, in particular for the treatment of infectious diseases.
Figures


References
-
- Fuller R. S., Brake A. J., Thorner J. (1989) Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science 246, 482–486 - PubMed
-
- van de Ven W. J., Voorberg J., Fontijn R., Pannekoek H., van den Ouweland A. M., van Duijnhoven H. L., Roebroek A. J., Siezen R. J. (1990) Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol. Biol. Rep. 14, 265–275 - PubMed
-
- Steiner D. F. (1998) The proprotein convertases. Curr. Opin. Chem. Biol. 2, 31–39 - PubMed
-
- Seidah N. G. (2011) What lies ahead for the proprotein convertases? Ann. N.Y. Acad. Sci. 1220, 149–161 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

