Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: comparison of SQUID-detected magnetic relaxometry and MRI
- PMID: 22539401
- PMCID: PMC3883306
- DOI: 10.1002/cmmi.499
Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: comparison of SQUID-detected magnetic relaxometry and MRI
Abstract
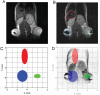
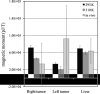
Both magnetic relaxometry and magnetic resonance imaging (MRI) can be used to detect and locate targeted magnetic nanoparticles, noninvasively and without ionizing radiation. Magnetic relaxometry offers advantages in terms of its specificity (only nanoparticles are detected) and the linear dependence of the relaxometry signal on the number of nanoparticles present. In this study, detection of single-core iron oxide nanoparticles by superconducting quantum interference device (SQUID)-detected magnetic relaxometry and standard 4.7 T MRI are compared. The nanoparticles were conjugated to a Her2 monoclonal antibody and targeted to Her2-expressing MCF7/Her2-18 (breast cancer cells); binding of the nanoparticles to the cells was assessed by magnetic relaxometry and iron assay. The same nanoparticle-labeled cells, serially diluted, were used to assess the detection limits and MR relaxivities. The detection limit of magnetic relaxometry was 125 000 nanoparticle-labeled cells at 3 cm from the SQUID sensors. T(2)-weighted MRI yielded a detection limit of 15 600 cells in a 150 µl volume, with r(1) = 1.1 mm(-1) s(-1) and r(2) = 166 mm(-1) s(-1). Her2-targeted nanoparticles were directly injected into xenograft MCF7/Her2-18 tumors in nude mice, and magnetic relaxometry imaging and 4.7 T MRI were performed, enabling direct comparison of the two techniques. Co-registration of relaxometry images and MRI of mice resulted in good agreement. A method for obtaining accurate quantification of microgram quantities of iron in the tumors and liver by relaxometry was also demonstrated. These results demonstrate the potential of SQUID-detected magnetic relaxometry imaging for the specific detection of breast cancer and the monitoring of magnetic nanoparticle-based therapies.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures


References
-
- Hellquist BN, Duffy SW, Abdsaleh S, Björneld L, Bordás P, Tabár L, Viták B, Zackrisson S, Nyström L, Jonsson H. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2011;117(4):714–22. - PubMed
-
- Kopans DB. Breast Imaging. Lippincott Williams & Wilkins; Philadelphia: 2007.
-
- Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. - PubMed
-
- Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257(1):246–53. - PubMed
-
- Weinstein S, Rosen M. Breast MR imaging: current indications and advanced imaging techniques. Radiol Clin North Am. 2010;48(5):1013–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
