MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes
- PMID: 22539765
- PMCID: PMC3380624
- DOI: 10.1161/CIRCRESAHA.112.269035
MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes
Abstract
Rationale: Repopulation of the injured heart with new, functional cardiomyocytes remains a daunting challenge for cardiac regenerative medicine. An ideal therapeutic approach would involve an effective method at achieving direct conversion of injured areas to functional tissue in situ.
Objective: The aim of this study was to develop a strategy that identified and evaluated the potential of specific micro (mi)RNAs capable of inducing reprogramming of cardiac fibroblasts directly to cardiomyocytes in vitro and in vivo.
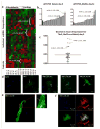
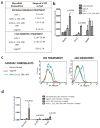
Methods and results: Using a combinatorial strategy, we identified a combination of miRNAs 1, 133, 208, and 499 capable of inducing direct cellular reprogramming of fibroblasts to cardiomyocyte-like cells in vitro. Detailed studies of the reprogrammed cells demonstrated that a single transient transfection of the miRNAs can direct a switch in cell fate as documented by expression of mature cardiomyocyte markers, sarcomeric organization, and exhibition of spontaneous calcium flux characteristic of a cardiomyocyte-like phenotype. Interestingly, we also found that miRNA-mediated reprogramming was enhanced 10-fold on JAK inhibitor I treatment. Importantly, administration of miRNAs into ischemic mouse myocardium resulted in evidence of direct conversion of cardiac fibroblasts to cardiomyocytes in situ. Genetic tracing analysis using Fsp1Cre-traced fibroblasts from both cardiac and noncardiac cell sources strongly suggests that induced cells are most likely of fibroblastic origin.
Conclusions: The findings from this study provide proof-of-concept that miRNAs have the capability of directly converting fibroblasts to a cardiomyocyte-like phenotype in vitro. Also of significance is that this is the first report of direct cardiac reprogramming in vivo. Our approach may have broad and important implications for therapeutic tissue regeneration in general.
Figures


Comment in
-
Direct reprogramming for cardiac regeneration: from dream to reality.Circ Res. 2012 May 25;110(11):1392-4. doi: 10.1161/CIRCRESAHA.112.270637. Circ Res. 2012. PMID: 22628569 Free PMC article. No abstract available.
References
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. - PubMed
-
- Nelson TJ, Martinez-Fernandez A, Terzic A. Induced pluripotent stem cells: Developmental biology to regenerative medicine. Nat Rev Cardiol. 2010;7:700–710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

